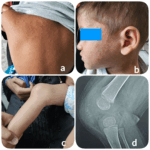
Every year, the ACR conducts a case-based image competition. Images are submitted for peer review. The winning images are displayed at ACR Convergence and winners receive other benefits, as well. This year, the overall winning images were submitted by Madhuri Challa, MD, of Hyderabad, Telangana, India. Presentation These are the representative clinical and radiological images…