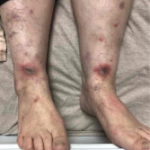
Meta-analyses have not shown an increased risk of other pulmonary events or death due to MTX. MTX rhinitis may develop in treated patients and may progress to respiratory failure. MTX should be stopped, and patients appropriately managed.
Other News
In other news, 35 mg delayed-release risedronate sodium tablets (Atelvia) are now available to treat post-menopausal osteoporosis.6 Tablets for weekly administration are available in a package of four. the rheumatologist
ad goes here:advert-1
ADVERTISEMENT
SCROLL TO CONTINUE
Michele B. Kaufman, PharmD, CGP, RPh, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
References
- Genetics and Biosimilars Initiative (GaBI). Clinical trials for etanercept biosimilars. 22 May 2015.
- Regeneron Pharmaceuticals Inc. News Release: Regeneron and Sanofi announce positive topline results from Phase 3 studies with sarilumab in patients with rheumatoid arthritis. 21 May 2015.
- Camus P, Faucher P. Pneumotox online: Drug-induced pulmonary disease database, v. 2. 2012.
- Highland K. Session IV, Presentation No. #3. Presented at American College of Rheumatology State-of-the-Art Clinical Symposium. May 2–3, 2015. Chicago.
- Pulawski S. Certain therapies linked to pulmonary toxicity among patients with rheumatic diseases. Healio. 20 May 2015.
- Han DH. Teva launches generic Atelvia. MPR. 19 May 2015.