A 59-year-old woman with rheumatoid arthritis (RA) presented to our pulmonary clinic for progressively worsening dyspnea of five years’ duration. She described progressively worsening dyspnea after a few minutes of walking on level ground. In addition, she noted worsening pain and morning stiffness of the wrists, knees and metacarpophalangeal (MCP) joints, with subcutaneous nodules. She denied fever, weight loss, cough, chest pain, palpitations and orthopnea.
She was diagnosed with RA seven years earlier and initially treated with prednisone, followed by adalimumab and methotrexate. Subsequently, her therapy was changed to tocilizumab monotherapy, which had been administered for the prior four years.
Four years before presenting to our clinic, she had also been diagnosed with chronic obstructive pulmonary disease (COPD) on the basis of abnormal pulmonary function tests. She had a five pack-year smoking history, but had quit smoking more than 20 years before. For the COPD, she was treated with inhaled tiotropium, fluticasone/salmeterol and albuterol, but experienced little symptomatic relief.
An examination revealed bilateral, faint inspiratory crackles with no wheezes or rhonchi. The musculoskeletal exam was remarkable for warm, tender and boggy wrists, MCP and knee joints. A serologic evaluation was initiated, and 40 mg of prednisone daily was started.
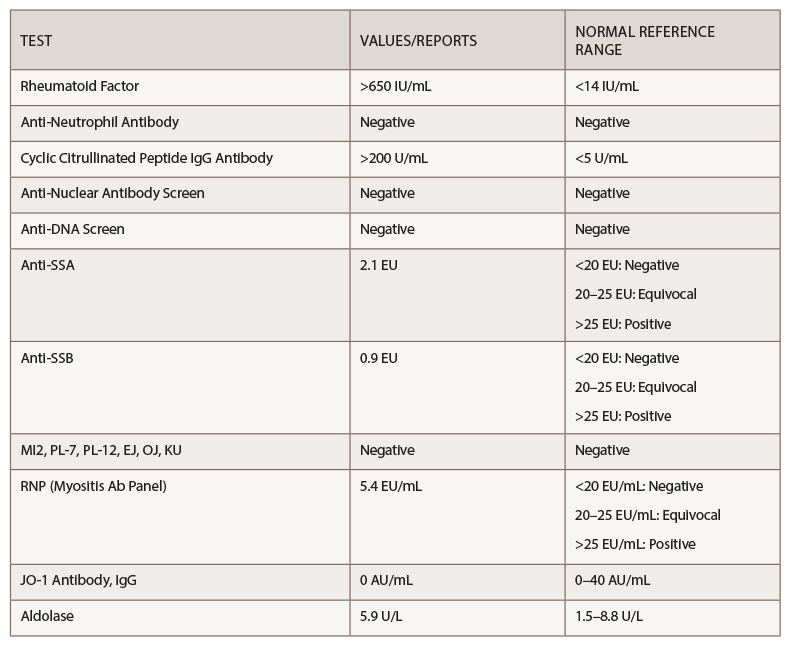
Her rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) were above the level of detection, at >650 IU/mL and >200 U/mL, respectively (see Table 1).
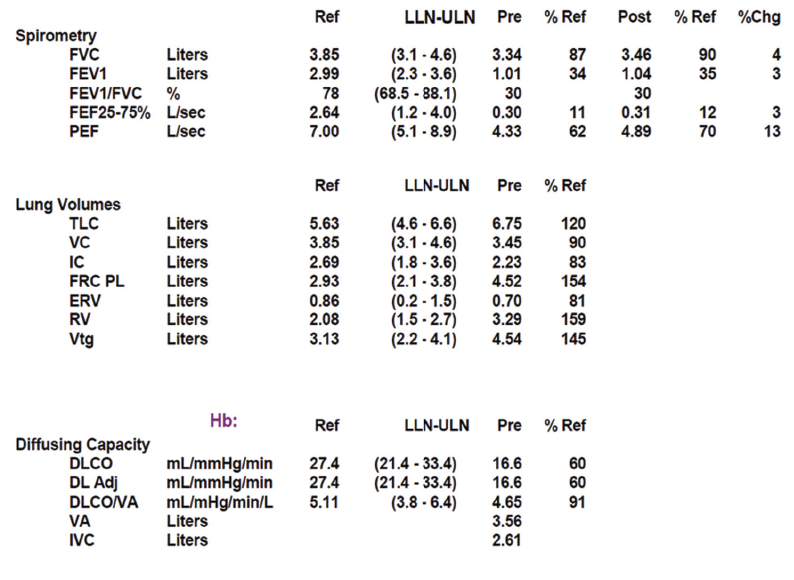
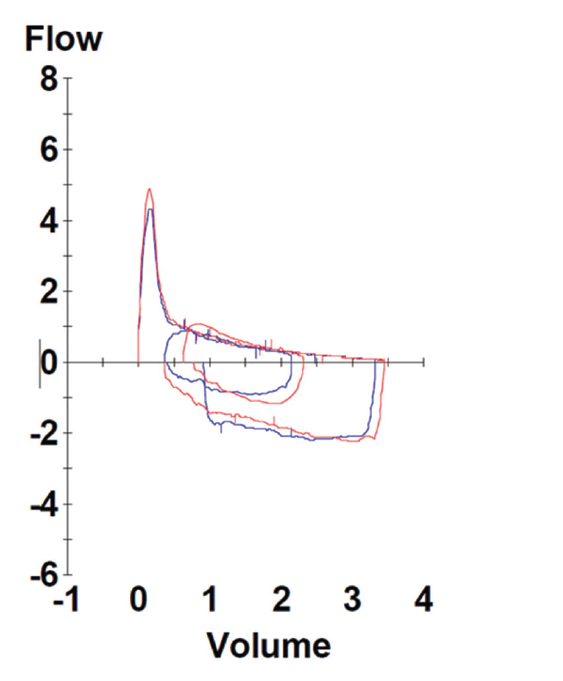
Pulmonary function testing revealed severe airway obstruction with no response to bronchodilators, markedly increased residual volume, suggestive of significant air trapping, and relatively preserved diffusion capacity for carbon monoxide (DLCO) (see Figures 1A and B, below). High-resolution computed tomography (HRCT) lung scanning with expiratory imaging revealed hyperinflation, diffuse bronchial thickening and mosaic attenuation in the areas of airway abnormality suggesting air trapping (see Figures 2A and B). She was subsequently diagnosed with obliterative bronchiolitis associated with RA (OB-RA).
Discussion
Epidemiology: RA is a common systemic inflammatory disorder of unknown etiology with a prevalence of about 1% of the general population.1 Extra-articular manifestations are common, leading to progressive disability, and are associated with increased mortality and socioeconomic costs. Pulmonary complications of RA are numerous and include interstitial lung disease, bronchiectasis/chronic bronchitis, pulmonary vasculitis, necrobiotic lung nodules and pleural effusion/fibrosis. Together, these are a significant cause of death in patients with RA.2,3
Obliterative bronchiolitis (OB), also known as bronchiolitis obliterans or constrictive bronchiolitis, is a rare pulmonary manifestation of RA presenting as severe obstructive small-airway disease. The occurrence of OB is not unique to RA and has been found in various clinical settings, including toxic inhalation, Sjögren’s syndrome, and following pulmonary and stem cell transplantation, suggesting OB is a final common pathway of airway destruction rather than a distinct clinical entity.4
OB following transplantation (OB-transplant) has been the subject of considerable study, but knowledge about OB in non-transplant patients remains limited. Obliterative bronchiolitis in patients with RA was first reported by Geddes et al. in 1977, with subsequently published literature about this entity being predominantly individual case reports and case series.5
Etiopathogenesis: Obliterative bronchiolitis in RA was initially believed to be a complication of penicillamine and gold; however, arguing against this hypothesis is the fact that OB-RA still occurs in the present time, when penicillamine and gold are no longer used. Moreover, because most of the patients in the two largest case series were non-smokers, it is unlikely that OB-RA is a manifestation of typical COPD.6,7
It is now believed that OB reflects an autoimmune manifestation of RA, although evidence to support this hypothesis is sparse.4 Based on the study by Sweatman et al., the expression of human leukocyte (HLA) antigens B40 and DR1 is increased in OB-RA, but not in the non-RA OB population, suggesting a genetic predisposition for OB-RA.8 Induction of autoimmune response to airway proteins, such as collagen V and K-α 1 tubulin, has been recognized as important in the pathogenesis of OB in lung transplant patients.9,10 Whether similar autoantibodies play a role in the development of OB in non-transplant patients is unknown.
Clinical Presentation: Obliterative bronchiolitis in RA is noted to have a female preponderance, with the mean age at diagnosis in the sixth or seventh decade of life, possibly reflecting the higher prevalence of RA in this age group.
The most common presenting symptoms are chronic dyspnea, bronchorrhea and cough.6,7 The reported interval between the diagnosis of RA and the onset of OB is 10–15 years.6,7 It is unclear whether this delay in diagnosis is attributable to the insidious nature of the disease or a lack of clinical recognition. Additionally, some patients with OB-RA present initially with only respiratory symptoms; the characteristic musculoskeletal findings occur later.
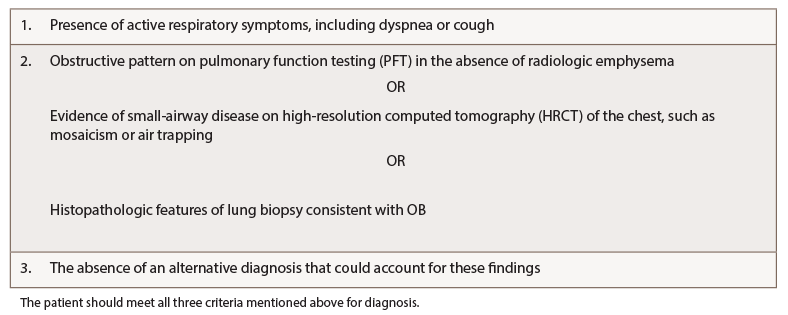
Diagnostic Evaluation: Lack of knowledge about OB-RA or well-accepted diagnostic criteria make correct diagnosis challenging. In 2018, Lin et al. proposed diagnostic criteria for OB derived from lung transplant patients, defining OB as the presence of active respiratory symptoms, including dyspnea or cough, in the presence of histological features consistent with OB or pulmonary function testing and/or imaging evidence of obstruction in the absence of an alternative diagnosis (see Table 2, right).6,11
Spirometry in OB-RA reveals an obstructive impairment with poor response to bronchodilators. In particular, measures of small-airway function, such as forced-expiratory flow (FEF) 25–75, are markedly reduced. In addition, lung volume analysis typically shows air trapping evidenced by increased residual volume but a fairly preserved diffusion capacity for carbon monoxide compared with emphysema patients with a similar impairment.
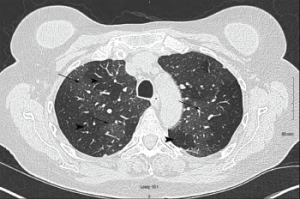
Figure 2a: Transverse Section
Arrowheads indicate areas of hyperlucency, suggesting air trapping, compared with hypolucent areas, indicated by arrows.
Chest radiography typically reveals clear lungs, often with hyperinflation, but HRCT of the chest is the diagnostic imaging modality of choice. Classic HRCT findings of OB-RA are mosaic attenuation and hyperinflation. Mosaic attenuation is defined as patchy areas of hyperlucency due to air trapping from bronchiolar obstruction and is best recognized during expiration (e.g., dynamic phase HRCT). Other common HRCT findings are bronchial wall thickening, cylindrical bronchiectasis and centrilobular pulmonary nodules.12
Invasive diagnostic methods are not essential to the diagnosis of OB-RA, but do help exclude alternative diagnoses. OB is characterized histologically by destruction of the bronchiolar wall by granulation tissue, effacement of the lumen, fibroproliferation and eventual replacement of the bronchiole by fibrous tissue. Lymphocytic infiltration of the bronchiolar wall has been described, but its significance is unknown. Surgical lung biopsy can provide a definite pathological diagnosis in most cases, but has significant attendant morbidity.
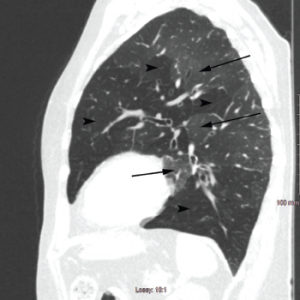
Figure 2b: Sagittal Section
Arrowheads indicate areas of hyperlucency, suggesting air trapping, compared with hypolucent areas, indicated by arrows.
Bronchoscopic methods, including transbronchial biopsies, are insensitive and are generally done to exclude other etiologies rather than make a diagnosis, as reported by Lin et al., where the diagnostic yield of transbronchial biopsy was only 28% vs. 100% for surgical biopsy.6 Similarly, Devouassoux et al. found histological pattern of constrictive bronchiolitis in eight of nine patients with OB-RA by surgical lung biopsy.7
Although the bronchoalveolar lavage (BAL) finding of neutrophilia has been reported in 10 of 12 patients with OB-RA and has been correlated with increasing disease severity in OB-transplant patients, the current level of published evidence is inadequate for definitive statements regarding diagnostic utility.
Outcome & Natural Progression: The natural progression of OB-RA is variable. The initial report of OB-RA in 1977 by Geddes et al. described a rapidly progressive disease, with five of six patients dying within 18 months.5
In contrast, Devouassoux et al. reported a mean interval of 19±32 months between symptom onset and diagnosis among 25 patients with OB-RA.7 Follow-up for more than four years showed 52% had symptomatic worsening, 32% had no change, 40% had chronic respiratory failure requiring oxygen supplementation, 4% underwent single lung transplantation, 16% died of respiratory failure, 16% had right-sided heart failure, and 100% had pulmonary hypertension on echocardiography.
Another case series that followed 32 patients for more than five years showed a 27% all-cause mortality, with an additional 15% requiring oxygen supplementation and 3% requiring lung transplantation.6
Management: There is no standard treatment strategy for OB-RA, and despite extensive research, there is no proven treatment modality for OB-transplant.13-16 Nonetheless, growing evidence indicates early use of azithromycin in lung transplant patients may decrease the occurrence of OB, possibly via an immunomodulatory effect.17 Whether azithromycin treatment is beneficial in OB-RA is unknown.
In the absence of reliable studies, and given the putative pathogenesis of OB-RA, aggressive anti-inflammatory and biologic therapy, typically used to treat RA, is often employed in the treatment of OB-RA.
Thus, treatment of OB-RA can be divided into two categories:
- (i) Symptomatic treatment of airflow obstruction with inhaled bronchodilators: Despite the airway obstruction of OB-RA tending to be unresponsive to bronchodilators, these are recommended for symptom management; and
- (ii) Treatment of the underlying pathology and pathogenesis that focuses on RA: Options include macrolides, often with additional immunosuppressive therapy with steroids, etanercept, azathioprine or cyclophosphamide.6,18-23 Because OB is characterized by irreversible damage to the airways, it is unclear if aggressive treatment of RA can stop disease progression.
Take-Home Messages
OB is a rare pulmonary manifestation of RA, presenting as severe obstructive small-airway disease with dyspnea, bronchorrhea and cough.
The etiopathogenesis of OB-RA is not fully understood and is believed to be directly related to an autoimmune manifestation of RA.
OB-RA has a female preponderance, with mean age at diagnosis in the sixth or seventh decade. The mean interval between the diagnosis of RA and OB is around 10–15 years.
A multidisciplinary team approach, involving rheumatology, radiology and pulmonology, is essential to an accurate diagnosis and for subsequent management. Proposed diagnostic criteria include: a) the presence of active respiratory symptoms, including dyspnea or cough; b) evidence of airway obstruction noted on pulmonary function tests, imaging or histological features consistent with OB; and c) exclusion of alternative diagnosis.
PFTs classically show reduced FEV1/FVC ratio, increased residual volume and fairly preserved DLCO. HRCT is the imaging test of choice with the classic presentation of mosaic attenuation and hyperinflation. If biopsy is considered, then surgical biopsy has a better diagnostic yield compared to transbronchial biopsy.
There is no validated treatment strategy for OB-RA. Management can be divided into two broad categories: i) treatment of airway obstruction with bronchodilators and ii) treatment of underlying pathology with immunomodulator and/or immunosuppressive therapy.
 Robin Paudel, MD, is a fellow in the Pulmonary and Critical Care Department at the University of Kentucky. He did his residency in internal medicine at Jersey City Medical Center, N.J., and earned his medical degree from Manipal College of Medical Sciences, Nepal.
Robin Paudel, MD, is a fellow in the Pulmonary and Critical Care Department at the University of Kentucky. He did his residency in internal medicine at Jersey City Medical Center, N.J., and earned his medical degree from Manipal College of Medical Sciences, Nepal.
 Prerna Dogra, MD, obtained her medical degree from Topiwala National Medical College, India. Her internal medicine residency was at Jersey City Medical Center. She is currently an assistant professor of medicine at the University of Kentucky.
Prerna Dogra, MD, obtained her medical degree from Topiwala National Medical College, India. Her internal medicine residency was at Jersey City Medical Center. She is currently an assistant professor of medicine at the University of Kentucky.
 Richard Scott Morehead, MD, is a professor of pulmonary and critical care medicine at the University of Kentucky. He received his medical degree from Oral Roberts University School of Medicine. He performed his residency at Emory University before completing his fellowship in pulmonary and critical care at the University of Alabama Medical Center.
Richard Scott Morehead, MD, is a professor of pulmonary and critical care medicine at the University of Kentucky. He received his medical degree from Oral Roberts University School of Medicine. He performed his residency at Emory University before completing his fellowship in pulmonary and critical care at the University of Alabama Medical Center.
References
- Silman A, Pearson J. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002;4 Suppl 3:S265–S272.
- Sihvonen S, Korpela M, Laippala P, et al. Death rates and causes of death in patients with rheumatoid arthritis: A population-based study. Scand J Rheumatol. 2004;33(4):221–227.
- Gonzalez A, Icen M, Kremers HM, et al. Mortality trends in rheumatoid arthritis: The role of rheumatoid factor. J Rheumatol. 2008 Jun;35(6):1009–1014.
- Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014 May 8;370(19):1820–1828.
- Geddes DM, Corrin B, Brewerton DA, et al. Progressive airway obliteration in adults and its association with rheumatoid disease. Q J Med. 1977 Oct;46(184):427–444.
- Lin E, Limper AH, Moua T. Obliterative bronchiolitis associated with rheumatoid arthritis: Analysis of a single-center case series. BMC Pulm Med. 2018 Jun 22;18(1):105.
- Devouassoux G, Cottin V, Liote H, et al. Characterisation of severe obliterative bronchiolitis in rheumatoid arthritis. Eur Respir J. 2009 May;33(5):1053–1061.
- Sweatman MC, Markwick JR, Charles PJ, et al. Histocompatibility antigens in adult obliterative bronchiolitis with or without rheumatoid arthritis. Dis Markers. 1986 Jun;4(1–2):19–26.
- Wilkes DS. Autoantibody formation in human and rat studies of chronic rejection and primary graft dysfunction. Semin Immunol. 2012 Apr;24(2)131–135.
- Hachem RR, Tiriveedhi V, Patterson GA, et al. Antibodies to K-α 1 tubulin and collagen v are associated with chronic rejection after lung transplantation. Am J Transplant. 2012 Aug;12(8):2164–2171.
- Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: An update of the diagnostic criteria. J Heart Lung Transplant. 2002 Mar;21(3):297–310.
- Tanaka N, Kim JS, Newell JD, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 2004 Jul;232(1):81–91.
- Watanabe S, Ishiyama K, Saeki K, et al. Tamibarotene for the treatment of bronchiolitis obliterans associated with chronic graft-vs-host disease. Chest. 2019 Jan;155(1):e1–e4.
- Vos R, Eynde RV, Ruttens D, et al. Montelukast in chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2019 May;38(5)516–527.
- Yadav H, Peters SG, Keogh KA, et al. Azithromycin for the treatment of obliterative bronchiolitis after hematopoietic stem cell transplantation: A systematic review and meta-analysis. Biol Blood Marrow Transplant. 2016 Dec;22(12):2264–2269.
- 16. Hachem R, Corris P. Extracorporeal photopheresis for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2018 Jul;102(7):1059–1065.
- Ruttens D, Verleden SE, Vandermeulen E, et al. Prophylactic azithromycin therapy after lung transplantation: Post hoc analysis of a randomized controlled trial. Am J Transplant. 2016 Jan;16(1):254–261.
- Hayakawa H, Sato A, Imokawa S, et al. Bronchiolar disease in rheumatoid arthritis. Am J Respir Crit Care Med. 1996 Nov;154(5):1531–1536.
- Yam LY, Wong R. Bronchiolitis obliterans and rheumatoid arthritis. Report of a case in a Chinese patient on d-penicillamine and review of the literature. Ann Acad Med Singapore. 1993 May;22(3):365–368.
- Penny WJ, Knight RK, Rees AM, et al. Obliterative bronchiolitis in rheumatoid arthritis. Ann Rheum Dis. 1982 Oct;41(5):469–472.
- Cortot AB, Cottin V, Miossec P, et al. Improvement of refractory rheumatoid arthritis-associated constrictive bronchiolitis with etanercept. Respir Med. 2005 Apr;99(4):511–514.
- Abhishek A, Gaywood I, Hubbard R. Case Reports (II) (546–574). Obliterative bronchiolitis complicating rheumatoid arthritis (RA) treated with etanercept. Rheumatology. 2008 Apr;47(suppl 2):ii158–ii165.
- van de Laar MAFJ, Westermann CJJ, Wagenaar SS, Dinant HJ. Beneficial effect of intravenous cyclophosphamide and oral prednisone on D‐penicillamine‐associated bronchiolitis obliterans. Arthritis Rheum. 1985 Jan;28(1):93–97.