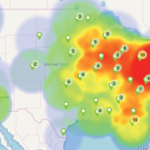
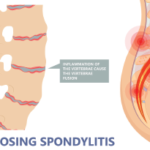
Rheumatologists play a critical role in healthcare delivery, especially with an aging U.S. population. Despite this, a workforce shortage exists—one that is projected to worsen.1,2 The ACR CareerConnection service is free to job seekers searching for opportunities in the field of rheumatology.3 This study examines currently available rheumatology employment opportunities across the U.S. The ACR…