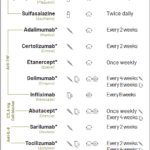
In summer 2024, two phase 3 studies were released with promising findings for the treatment of patients with systemic lupus erythematosus (SLE) and those with lupus nephritis. SLE Disease Activity Dapirolizumab pegol is a novel, investigational, Fc-free anti-CD40L agent for people living with moderate to severe SLE.1 The randomized, double-blind, parallel-group PHOENYCS GO trial (N=321)…