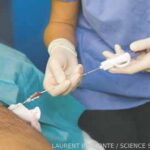
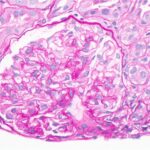
SAN DIEGO—As part of a Nov. 14 session on lupus nephritis at ACR Convergence 2023, Simone Appenzeller, MD, PhD, shared perspectives on the importance of biopsy to inform its diagnosis, prognosis and treatment, with an emphasis on childhood disease. Prompt diagnosis and treatment of lupus nephritis is perhaps even more important for children than for…