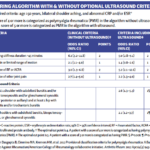
In an ACR Convergence 2022 session, Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery, New York City, discussed the use of sarilumab as a potential glucocorticoid-sparing therapy in a phase 3 study in patients with treatment-refractory polymyalgia rheumatica (PMR), one of the most common inflammatory diseases…