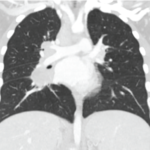
With the ongoing COVID‑19 pandemic, several tools and strategies have been developed and implemented to reduce the spread of disease. These include social distancing, adequate ventilation, masks, monoclonal antibody treatment and vaccination.1 As of December 2021, 60% of all Americans have been fully vaccinated, and 8 in 10 adults in the U.S. have received at…