Rheumatology practices can combat declining reimbursements with ancillary services. Experts discuss adding infusion, ultrasound & research services to boost revenue & patient care.

Tips for Recruiting & Onboarding Advanced Practice Providers
Experts share strategies for recruiting, onboarding & retaining APPs to address the rheumatologist shortage & improve patient access.

Independent Rheumatology Practices Face Challenges Amid Changing Landscape
At this Practice Innovation Summit session, experts discussed the challenges that come with surviving as an independent rheumatology practice in the current health environment.

8 Tips for Hiring and Keeping the Best Employees for Your Practice
Even if you hire human resources professionals, as a private practice owner, you still want to have a good grip on the basics of attracting great people to your practice and keeping them happy. Speakers at ACR Convergence 2025 shared pearls to help practice owners do just that.

Negotiating with Commercial Payers Takes Planning, Strategy
At the inaugural Practice Innovation Summit, two experts offered advice on negotiating insurance contracts.

Make All the Right Moves with Your Practice Financials
Taking the time to understand the financial stats of your practice could help you take steps necessary to improve the practice’s financial viability.
MIPS, MVPs & RISE: The Scoop on Quality Reporting for 2026
ACR experts break down MIPS, MVPs & the RISE registry, helping clinicians understand quality reporting requirements for 2026 & beyond.
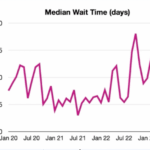
Workforce Solutions
Three experts discussed novel and practical strategies and resources that rheumatology clinics can use to improve the referral process, screening times and patients access to care.
The ACR Revamps RISE Registry into a Productivity & Learning Tool
The ACR’s new RISE Registry, set for 2026, will be a modern productivity tool with new analytics, easy EHR connection & robust research data.

The Pros & Cons of AI
As the uses for artificial intelligence grow within rheumatology, so do the benefits and the concerns about the technology. In a session at ACR Convergence 2025, two experts discuss the research on both sides of the debate.
- 1
- 2
- 3
- …
- 173
- Next Page »