With the U.S. FDA’s first approval of a biosimilar drug earlier this month, rheumatologists stress the need to ensure safety, efficacy of such drugs in treating rheumatic diseases
Search results for: biosimilars
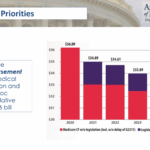
ACR Advocacy Update 2025, Federal & State
ACR representatives outlined its 2025 legislative priorities, including fixing the MPFS, reforming prior authorization & protecting telehealth reimbursement.
ACR Delegation to Address AI, Non-Compete Agreements at Interim AMA House of Delegates Meeting
The ACR’s resolutions at the Interim HOD meeting, held Nov. 14–18, will address non-compete agreements as well as the use of artificial intelligence (AI) in prior authorization processes.

Encourage Physical Activity During Rheumatic Disease Awareness Month
This year’s Rheumatic Disease Awareness Month encourages rheumatology professionals to collaborate with patients on physical activity routines that benefit their health.
UHC Formulary Change Impacts Coverage for Ustekinumab
Effective Sept. 1, the brand name drug Stelara will no longer be covered on the UnitedHealthcare commercial plan prescription drug list.

FDA Approves Ustekinumab-stba for All Forms & Dosages as Its Reference Product
Ustekinumab-stba (Steqeyma) now has FDA approval for all forms and dosages of its reference product, ustekinumab (Stelara), including a subcutaneous injection to treat pediatric patients with plaque psoriasis or psoriatic arthritis.

President’s Corner: The ACR Is on Your Side
Encouraging lawmakers to pass legislation and promote policies that positively affect rheumatologists, rheumatology care teams and those living with rheumatic disease is one of the most impactful ways the ACR advances its mission. The role of advocacy has never been more critical than in recent months, when we have seen an unprecedented number of executive…

Biosimilar Update: 2025 Brings More FDA Approvals & Interchangeability
Here is a brief recap of the latest FDA-approved biosimilar agents, highlighting their reference products and interchangeability.
The ACR Calls on CMS, OMB to Reduce Burdensome Healthcare Regulations
In response to agency requests for feedback on how to streamline regulations and reduce administrative burden on Medicare program stakeholders and small business owners, the ACR called for the removal of certain regulations related to prior authorization, pharmacy benefit managers and Medicare Part B and Part D access.
ACR Executive Committee Holds Meetings with Heads of FDA, CMS
As in past administrations, members of the Executive Committee scheduled meetings with leaders of the Food & Drug Administration and Centers for Medicare & Medicaid Services to discuss ACR policy priorities and agency agendas.
- « Previous Page
- 1
- …
- 4
- 5
- 6
- 7
- 8
- …
- 32
- Next Page »