Dr. Kumar links a traffic-jammed drive to healthcare challenges, exploring privilege, advocacy & resilience in this Rheuminations essay.


Dr. Kumar links a traffic-jammed drive to healthcare challenges, exploring privilege, advocacy & resilience in this Rheuminations essay.

Michael Cammarata, MD, RhMSUS |
Long-term use of corticosteroids is associated with numerous adverse effects, particularly in children, but guidance is lacking on how to manage low-dose prednisone in clinically quiescent disease. In The Great Debate at ACR Convergence 2025—Corticosteroids in Pediatric SLE: Slay or Stay, two pediatric lupus experts took to the stage to explore this important topic.

Michael Cammarata, MD, RhMSUS |
In the ACR Convergence 2025 session, Great Debate: Ultrasound vs. Temporal Artery Biopsy in Giant Cell Arteritis, two vasculitis experts went head to head to determine the ideal first test in the diagnosis GCA: ultrasound or biopsy.

Research demonstrating the effectiveness of subcutaneous anifrolumab-fnia as an auto-injector in patients with active systemic lupus erythematosus is the basis of a recent decision in the EU to recommend the treatments approval for this patient population.
Jason Liebowitz, MD |
Review expert strategies & clinical pearls for managing SLE & lupus nephritis. Get therapeutic updates from the ACR’s 2025 Review Course.

Graciela S. Alarcón, MD, MPH, MACR |
This review highlights some of the many abstracts on lupus nephritis research presented at ACR Convergence 2025. They demonstrate advances made on early recognition of the condition, as well as offering some hope in terms of achieving better outcomes.

At the Dubois Memorial Lecture presented at ACR Convergence 2025, Shaun Jackson, MD, PhD, discussed the evolving understanding of the role of B cells in SLE.
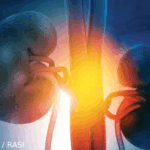
Lupus nephritis is one of the leading causes of mortality for patients with systemic lupus erythematosus (SLE), and patients with both SLE and end-stage renal disease have standardized mortality ratios more than 60 times that of patients with SLE with normal kidney function.1 The good news: Rheumatologists now have not one, but two approved options…
In summer 2024, two phase 3 studies were released with promising findings for the treatment of patients with systemic lupus erythematosus (SLE) and those with lupus nephritis. SLE Disease Activity Dapirolizumab pegol is a novel, investigational, Fc-free anti-CD40L agent for people living with moderate to severe SLE.1 The randomized, double-blind, parallel-group PHOENYCS GO trial (N=321)…

In late July 2022, the U.S. Food & Drug Administration (FDA) approved belimumab (Benlysta) for the treatment of children with active lupus nephritis aged 5 to 17 years old receiving standard therapy.1 Despite recent advances in treatment options for patients with systemic lupus erythematosus (SLE), those with kidney involvement may develop endstage renal disease and…