(Reuters)—Flexion Therapeutics Inc. said its injectable drug to treat osteoarthritis-related knee pain was approved by the U.S. Food and Drug Administration. The approval comes at a time when U.S. federal authorities are implementing a slew of measures to combat opioid abuse, with President Donald Trump in August declaring the opioid epidemic a national emergency. The…
Search results for: opioid
Cigna to End OxyContin Painkiller Coverage, Signs Contract for Alternative
(Reuters)—Amid a growing U.S. opioid addiction, health insurer Cigna Corp will stop covering OxyContin, the opioid painkiller sold by Purdue Pharma LP, as of January 1 and will instead cover an equivalent with a formulation less vulnerable to abuse, the company said on Wednesday. The insurer has signed a “value-based contract” with Collegium Pharmaceutical Inc…
Washington State Sues OxyContin Maker Purdue Pharma
(Reuters)—Washington state on Thursday sued OxyContin maker Purdue Pharma LP, becoming the latest state or local government to file a lawsuit seeking to hold pharmaceutical companies accountable for a national opioid addiction epidemic. The city of Seattle also filed a separate lawsuit against Purdue as well as units of Teva Pharmaceutical Industries Ltd, Johnson and…
Many Drug Companies Fail to Conduct Timely Safety Checks on Medicines after FDA Approval
(Reuters Health)—In the rush to approve new medicines, the U.S. Food and Drug Administration often requires drug companies to study possible side effects and alternative doses for medicines once they hit the broader market. A September 20 online analysis in the New England Journal of Medicine concludes that, in many cases, that’s not being done….
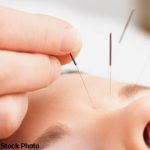
Acupuncture & Electrotherapy May Help Patients after Total Knee Arthroplasty
A new review examined how drug-free interventions affect pain relief and analgesic consumption for patients who have had knee surgery. Although little evidence shows these treatments reduce actual pain, electrotherapy and acupuncture may help patients delay their postoperative use of opioids…
S.C. Sues Purdue, Maker of OxyContin, Over Deceptive Marketing
(Reuters)—On Tuesday, South Carolina sued Purdue Pharma LP, becoming the latest state or local government to accuse the OxyContin maker of deceptive marketing practices that have contributed to a national opioid addiction epidemic. The lawsuit by South Carolina Attorney General Alan Wilson, filed in Richland County Court of Common Pleas in Columbia, accuses the company…

Rheumatology Drug Updates: Opana ER Painkiller Pulled from U.S. Market; Upadacitinib to Treat RA, and More
Opana ER Pulled from U.S. Market Last month, the U.S. Food and Drug Administration (FDA) asked Endo Pharmaceuticals to remove oxymorphone hydrochloride extended release (Opana ER) from the U.S. market due to public health consequences related to abuse. The agency has concerns that the risks presented by the treatment do not outweigh its benefits.1 On…
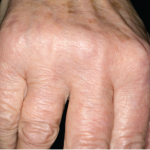
Effectiveness of Retinoic Acid, Hydroxychloroquine Examined for Hand OA
The Osteoarthritis Research Society International (OARSI) held the 2017 OARSI World Congress in Las Vegas, April 27–30. Below, we report on two of the sessions held. Retinoic Acid & Hand Osteoarthritis Retinoic acid is a vitamin A derivative and hormonal signaling molecule with a role in cartilage and skeletal development. Retinoic acid has complex function,…
As Drug Prices Drop, Generics Makers Fight Back with Deals
NEW YORK (Reuters)—Generic drug makers are turning to mergers and acquisitions to shield themselves against a concerted effort by U.S. regulators to crack down on steep drug prices. Impax Laboratories Inc, Perrigo Company Plc and Alvogen Inc have been talking to advisers about strategic options for their generics businesses, ranging from acquisitions to increase scale…
Progress Made During HOD Annual Meeting
The AMA House of Delegates (HOD) met in Chicago for its annual meeting June 9–14. More than 530 HOD members were present, with several hundred AMA and association staff and guests. As I am sure you have noted from past issues of ACR@Work, the ACR is in its five-year membership review. The ACR must be…
- « Previous Page
- 1
- …
- 18
- 19
- 20
- 21
- 22
- …
- 32
- Next Page »