In a session at EULAR 2025, Dr. Laura Coates discussed the management of PsA, providing insights into the current research and when clinicians may want to consider prescribing specific medications.
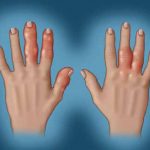

In a session at EULAR 2025, Dr. Laura Coates discussed the management of PsA, providing insights into the current research and when clinicians may want to consider prescribing specific medications.

Ustekinumab-stba (Steqeyma) now has FDA approval for all forms and dosages of its reference product, ustekinumab (Stelara), including a subcutaneous injection to treat pediatric patients with plaque psoriasis or psoriatic arthritis.
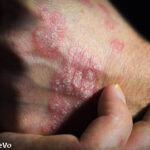
Results from two studies found that deucravacitinib improved the signs and symptoms of patients with psoriatic arthritis who were biologic disease-modifying anti-rheumatic drug naive and those previously treated with a tumor necrosis factor α inhibitor.

The FDA has approved bimekizumab-bkzx for the treatment of adults with psoriatic arthritis, non-radiographic axial spondyloarthritis and ankylosing spondylitis.
Philip Mease, George Reed, Alexis Ogdie, Dimitrios A. Pappas, & Joel M. Kremer |
According to a new study, fibromyalgia prevalence is elevated in PsA and is associated with elevated disease measures, confounding reliable disease assessment for treat-to-target goals.
Shashank Cheemalavagu, MD, Yuxuan Jin, MS, & M. Elaine Husni, MD, MPH |
Objective We aimed to identify clinical and demographic features associated with the interval between the appearance of psoriasis and the onset of psoriatic arthritis (PsA). Methods We identified patients with psoriasis and PsA diagnoses from our tertiary care psoriatic disease biorepository: a longitudinal, real-world database including clinical information and patient-reported outcomes. We used a multivariable,…

David S. Pisetsky, MD, PhD |
Editor’s note: What research on psoriatic arthritis (PsA) presented at ACR Convergence 2024 has the greatest potential for a positive impact on clinical care, treatment options or serve as the basis for future research? That’s the question The Rheumatologist asked David S. Pisetsky, MD, PhD—our founding editor—to consider. Dr. Pisetsky, a professor of medicine and immunology…

Experts address how sex differences affect the clinical presentation, diagnosis, response to therapy and experience of illness for patients with PsA.

Experts shared insights into their work on building consensus for the use of musculoskeletal ultrasound for the diagnosis and management of RA and PsA.

WASHINGTON, D.C.—As of November 2024, there are 16 biologic disease-modifying anti-rheumatic drugs (bDMARDs) that are FDA approved for the treatment of psoriatic arthritis (PsA). Incredible news, right? But as my fellowship program director used to say, “There’s no free lunch.” This buffet of options is excellent for our patients, but poses challenges to the practicing…