Pathophysiology
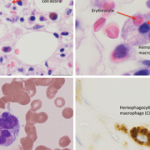
The pathological mechanisms of MAS are not fully understood. In clinically similar primary HLH, the uncontrolled proliferation of T cells and macrophages has been linked to decreased natural killer (NK) cell and cytotoxic T cell function, often due to mutations in the gene encoding perforin.8,10 Perforin is a protein that cytolytic cells utilize to induce apoptosis of target cells, such as tumor cells or cells infected by viruses. In a sizable proportion of patients with primary HLH, the disease is caused by mutations in another gene, MUNC13-4.11 The protein encoded by the MUNC13-4 gene is involved in the release of perforin into the immune synapse with a target cell. Although the cytolytic cells of patients with MUNC13-4 mutations produce sufficient amounts of perforin, their ability to kill target cells is greatly diminished. Defects in the granule-dependent cytotoxic functions of lymphocytes have also been implicated in several other genetic diseases associated with the hemophagocytic syndrome.
Recent observations suggest that as in HLH, MAS patients have profoundly depressed NK cell function, often associated with abnormal perforin expression, and these abnormalities are associated with specific MUNC13-4 and perforin gene polymorphisms.12,13 Although the exact pathways that could link cytolytic abnormalities in these patients with excessive expansion of macrophages are not clear, the presence of defects in the granule-dependent cytotoxic activity of lymphocytes in several diseases associated with hemophagocytic syndromes highlights the importance of this function in restoring the immune system to a state of equilibrium during some inflammatory responses.