(Reuters)—The U.S. Food & Drug Administration (FDA) is expected to announce a new warning on Johnson & Johnson’s (J&J’s) coronavirus vaccine related to a rare autoimmune disorder, The Washington Post reported on Monday, citing four people familiar with the matter.1
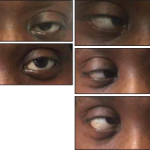
According to The Post, about 100 preliminary reports of Guillain-Barré syndrome have been detected in the U.S. after vaccination with J&J shot, mostly in men, many of whom were 50 or older. Around 12.8 million people have received the one-dose vaccine in the U.S.
J&J and the FDA were not immediately available for comment.
Guillain-Barré syndrome, in which the body’s immune system attacks the protective coating on nerve fibers, most often follows a bacterial or viral infection.
The condition has been linked in the past to vaccinations—most notably to a vaccination campaign during a swine flu outbreak in the U.S. in 1976, and decades later to the vaccine used during the 2009 H1N1 flu pandemic.
Reference
- McGinley L, Sun LH. FDA adds new warning on Johnson & Johnson vaccine related to rare autoimmune disorder. The Washington Post. 2021 Jul 12.