CHICAGO—Diagnosis concerns and limited treatment options for calcium pyrophosphate deposition (CPPD) disease were the topic of a discussion at ACR Convergence 2025.
What Is CPPD?
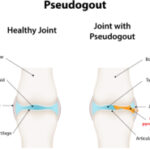
CPPD occurs when calcium pyrophosphate crystals form around chondrocytes (chondrocalcinosis) and deposit in cartilage, tendon and joint capsules. In the long term, the condition has inflammatory and mechanical consequences.
“Chondrocalcinosis is a radiographic finding and not the disease,” said Sara Tedeschi, MD, MPH, clinical investigator at Brigham and Women’s Hospital and an assistant professor of medicine at Harvard Medical School, Boston. “It becomes a disease when there are symptoms along with radiographic or tissue evidence of the crystals.”
CPPD disease typically affects adults older than 60. Joint trauma or surgery are risk factors, and CPPD disease can appear in conjunction with rheumatoid arthritis (RA), osteoarthritis (OA) and gout. Hemochromatosis, hyperparathyroidism, hypophospatasia and hypomagnesemia are risk factors, although they are uncommon. Genetic links have been found for early onset cases (i.e., younger than 30 years old). Just this year, a genome-wide association study identified ENPP1 as a causal pathway.
OA with CPPD is thought to be the most common symptomatic manifestation. In addition to the usual symptoms of OA, there is chondrocalcinosis on radiographs and effects seen in unusual joints, such as the shoulder.
Other manifestations include:
- Acute calcium pyrophosphate crystal arthritis, formerly called pseudogout;
- Chronic calcium pyrophosphate crystal arthritis, formerly called pseudo RA; and
- Crowned dens syndrome, which mimics meningitis.1
“These terms are an alphabet soup,” said Dr. Tedeschi. “We are hoping to standardize definitions across studies to identify groups of patients for enrollment in future clinical trials. Then we can develop targeted therapies for this condition that very much needs effective treatments.”
Diagnostic Difficulties
Radiology is important in treatment decisions. Conventional X-rays have a specificity of more than 90%, but the sensitivity is only about 50%. Ultrasound has a sensitivity of 85% and a specificity of 85%, allowing higher confidence the patient does have CPPD disease. Standard computed tomography (CT) and dual-energy CT have about twice the sensitivity of X-rays, plus the added advantage of quantifying the CPPD.
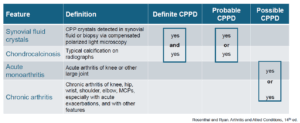
As of now, there is no validated clinical tool to diagnose CPPD disease. A 2016 study considered the probability of CPPD with combinations of synovial fluid crystals, chondrocalcinosis, acute monoarthritis and chronic arthritis. Patients were diagnosed as either definitely, probably or possibly having CPPD.2