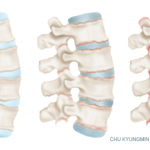
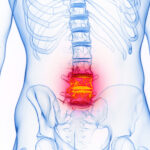
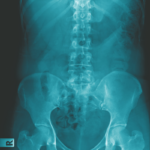
Axial spondyloarthritis (axSpA), comprising ankylosing spondylitis (AS) and nonradiographic axial SpA, is the main form of chronic inflammatory arthritis affecting the axial skeleton. In this resource center, you’ll find Dr. David Pisetsky’s picks for the top research in axSpA presented at ACR Convergence 2024 our summary of the 2019 treatment guideline, as well as current research reviews, case reports and clinical articles on advances in the diagnosis and management of axial spondyloarthritis.
FEATURED ARTICLE: Measures of Success