BOSTON—Diagnosis of both cardiac sarcoidosis and neurosarcoidosis requires a process of exclusion and a high degree of clinical suspicion, according to a presentation at the ACR/ARHP Annual Meeting in Boston in November 2014, Sarcoidosis in 2014. Akin to problems associated with the general diagnosis of sarcoidosis, there is no one reliable diagnostic test for either condition, the clinical manifestations can be nonspecific, and there are no evidence-based guidelines for treatment.
Daniel Culver, DO, a pulmonologist at the Cleveland Clinic, said diagnosis of cardiac sarcoidosis can be difficult and should include a finding of sarcoidosis in an organ outside the heart and presence of a heart lesion that cannot be explained by other causes. A patient’s symptoms, however, are still the most important for diagnosis.
“I ask my patients: ‘Are you having palpitations, are you having dizzy spells, have you passed out?’ If any of those things are true, you have to go looking hard for a diagnosis of cardiac sarcoidosis,” he said.
Patients with cardiac sarcoidosis can have congestive heart failure, impaired systolic function, ventricular dysfunction, conduction delays and reduced left ventricular ejection fraction. The symptoms can range from asymptomatic conduction abnormalities to fatal ventricular arrhythmias, depending on the location and extent of granulomatous inflammation.1
Screening for cardiac sarcoidosis should include not only an assessment of the patient’s symptoms, but also an electrocardiogram that can help detect any conduction system abnormalities and whether the heart is involved. Blood test abnormalities are not useful for diagnosis.
Imaging Modalities Used in Diagnosis
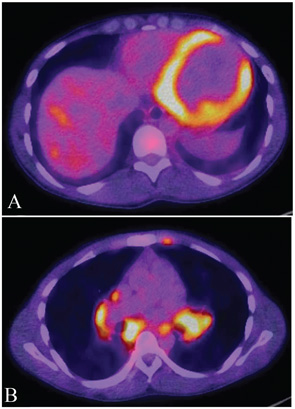
No gold standard has been established for imaging cardiac sarcoidosis, and the sensitivity and specificity of imaging modalities are limited. However, contrast-enhanced magnetic resonance imaging (MRI) “does have some utility,” Dr. Culver said. “When you see late enhancement on MRI, that’s a sign that the patient is likely to have some issue. Patients with late enhancement have a worse course.”
MRI will indicate the distribution of myocardial changes associated with cardiac sarcoidosis, with those changes most evident on the epicardium, the midmyocardium or the transmyocardium, Dr. Culver said. There are generally lymphocytes at the border zones around the granulomas, as well as a dense band of fibroblasts, collagen fibers and proteoglycans encasing an aggregate of inflammatory cells.1 Fluorodeoxyglucose (FDG) positron emission tomography (PET) has also been demonstrated to have value in diagnosis of myocardial inflammation.
Corticosteroids are considered effective treatment, although there are no guidelines that indicate the optimal dose or duration of therapy. Immunosuppressive therapies, such as methotrexate and infliximab, are potential treatments, but they are also not without risk. Steroid therapy can be tapered after a long course of immunosuppressant therapy.2
Cardiac sarcoidosis is associated with a poor prognosis, and it is not known whether treatment will improve survival, but “it might,” Dr. Culver said. “What we do know is that treatment that preserves ejection fraction goes better than treatment that doesn’t preserve ejection fraction.” Use of implantable cardioverter defibrillators (ICDs) has revolutionized treatment for patients with sustained ventricular tachycardia or ventricular fibrillation, but risk is attributed to ICDs in some patients, he said. Radiofrequency ablation can also be effective therapy, after courses of immunosuppressants and antiarrhythmics, but Dr. Culver’s data have shown that dysrhythmia still can occur. “It can be useful but all those patients should get an ICD as well,” Dr. Culver said.
Sarcoidosis in the CNS
Neurosarcoidosis (i.e., affecting the central nervous system [CNS]) is also a challenging heterogeneous disease, is relatively rare, usually occurs with other forms of sarcoidosis, and is one that can affect the brain, spinal cord, peripheral nerves and muscles, said Nadera J. Sweiss, MD, associate professor of medicine at the University of Illinois College of Medicine at Chicago. Similar to diagnostic problems associated with cardiac sarcoidosis, diagnosis of the condition requires exclusion of other disorders. Any recommendations about diagnosis and management are based on expert opinion rather than evidence-based trials, Dr. Sweiss said.
Manifestations of neurosarcoidosis can include facial nerve palsy (the most common manifestation), optic neuropathy, vestibulocochlear nerve involvement, trigeminal neuralgia, leptomeningeal inflammation, headache, seizures, cognitive dysfunction, parenchymal lesions, meningitis and hydrocephalus. Strokes can occur, but they are a rare complication, Dr. Sweiss said. Patients may report fatigue, memory loss, mood and sleep disturbances.
Because the clinical manifestations are diverse, diagnosis is challenging. MRI with or without contrast, FDG PET and analysis of cerebrospinal fluid (CSF) are helpful. Often, MRI of the brain will show a basilar leptomeningeal involvement. A definitive diagnosis can require a biopsy. Most important for diagnosis, according to Dr. Sweiss, is elevation of protein in CSF.
There are no established standards for treatment of neurosarcoidosis. Currently, the first-line standard of care is glucocorticosteroids, “despite the lack of conclusive data,” Dr. Sweiss advised. “DMARDs and TNF inhibitors are safe and effective, but remain investigational.”
Kathy L. Holliman, MEd, is a medical writer based in Beverly, Mass.