Meaningful Use may be changing but it is not going away—yet. Rheumatologists must sign up with two of three registries by Feb. 29 or pay significant financial penalties for not complying with this specific part of the Meaningful Use requirements. The three choices for registries include the Immunization Registry, the Syndromic Surveillance Registry and a specialized registry. The ACR’s RISE Registry meets the specialized registry specification.
The 2009 American Recovery and Reinvestment Act authorized the Centers for Medicare and Medicaid Services (CMS) to provide reimbursement incentives to providers who can demonstrate meaningful use of electronic health record (EHR) systems. The intent was to encourage providers and hospitals to adopt EHRs, implement systems locally in ways that fully realize the potential of these systems and use the systems to exchange data.
Implemented in stages, the modified objectives for Stage 2 during the years 2015–2017 include the requirement that the provider or hospital actively engage with a public health agency to submit electronic public health data. The imposition of a deadline for this requirement—and one so early in 2016—is surprising and unexpected.
According to Melissa Francisco, the ACR’s director of registries, the CMS has not yet released information clarifying the amount of the financial penalty that will be imposed on physicians who fail to sign up with two registries by Feb. 29.
In previous years, penalties for noncompliance with other parts of the program were dependent on the year that a physician began participating in the Meaningful Use program. The CMS has not specified, however, how the penalty will be assessed for failure to sign up for the registries by the end of February.
“If all you do this year is register and start working toward data submission, then that will count toward meeting the requirement,” Ms. Francisco said. “This is the first time I have seen a deadline pop up like this. Given that CMS does not offer partial credit for meeting Meaningful Use requirements, it is imperative that providers meet this requirement by the Feb. 29 deadline.”
RISE Registry
The RISE Registry is the ACR’s own specialized registry, participation is free, registration is simple, and participation in the RISE Registry meets the specialized registry option for this Meaningful Use measure.
Members simply need to reach out to the ACR at [email protected] and request a registration contract.
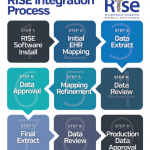
The ACR launched the RISE (Rheumatology Informatics System for Effectiveness) Registry in 2014, and it is an enhanced version of the Rheumatology Clinical Registry. RISE is an EHR-enabled registry that has captured data on more than 1 million patient encounters. It is one of the largest rheumatology patient registries in the country.
E. William St.Clair, MD, the ACR’s immediate past president, wrote in the June 16, 2015, issue of The Rheumatologist that the registry was designed to become a “best-in-class resource to help rheumatologists, rheumatology health professionals and researchers manage patient populations, improve patient care and navigate the ever-changing healthcare environment.” RISE gives physicians real-time access to data and customized quality-improvement quires and reports.
The RISE Registry collects demographic, medication, and lab data, as well as data on disease activity and functional status. The ACR is responsible for oversight and monitoring of the registry and its information integrity. The system allows participants to run both standard and custom queries for one’s own patient population and to compare that patient population with the aggregate of the patients in the registry.
The registry is HIPAA compliant, and the ACR will not have access to a patient’s protected health information. Any patient protected health information is stored separately. The only data used by the ACR or third parties for research or other purposes will be aggregate data, not individualized data.
The participation agreement includes both a data use agreement and a business associate agreement. The ACR does not require workflow changes to participate in the registry; it is acceptable if there are data that a rheumatologist practice is not capturing. Additionally, participation includes working with the ACR’s registry partner, FIGmd, over a series of conference calls to review and refine data mapping to the registry. More information about RISE is available at http://www.rheumatology.org/I-Am-A/Rheumatologist/Registries/RISE/RISE-FAQs.
Additional Registries
The Syndromic Surveillance Registry is operated by individual states. Please contact your state’s public health department for specific details.
The CDC offers a list of Immunization Registries by state. These include population-based data on immunization doses administered by participating providers. For more information, see http://www.cdc.gov/vaccines/programs/iis/contacts-registry-staff.html.
Regardless of which registries you choose, the date to remember is Feb. 29, 2016, or you’ll face financial penalties for not complying with the requirement.
Kathy L. Holliman, MEd, is a medical writer based in Beverly, Mass.