Telehealth is at risk. Congress must act before the end of September to maintain vital flexibility and reimbursement for telemedicine services.

ACR’s Response to Proposed Physician Medicare Cuts Garners National Media Attention
ACR President Carol Langford, MD, MHS, is making headlines as a leading voice in the fight against proposed Medicare reimbursement cuts that threaten the sustainability of rheumatology practices and patient access to care.
Share Your Story: Help Shape the Future of Noncompete Agreements for Rheumatologists
The Federal Trade Commission is seeking input about the use and impacts of noncompete agreements, with particular interest in the healthcare sector. ACR/ARP members are encouraged to share your experiences about how such agreements have affected your career and patients.
AHIP Confirms Continued Coverage of ACIP-Recommended Vaccines Through End of 2026
All vaccines recommended by the CDC’s ACIP)as of Sept. 1, 2025 — including updated COVID-19 and influenza shots — will be covered by health plans with no cost‐sharing for patients through the end of 2026.
2024 Traditional MIPS & MVP Final Scores Now Available
MIPS-eligible clinicians should review their scores to confirm performance. Any scoring concerns can be addressed through a targeted review

Oral Treatment for PsA Inches Closer to FDA Approval
The FDA will consider a supplemental new drug application for deucravacitinib for the treatment of adults with active psoriatic arthritis based on promising results from clinical trials.

A New Treatment for Fibromyalgia?
The FDA has approved a new drug application for TNX-102 SL (Tonmya), a tablet with 2.8 mg of cyclobenzaprine HCl, after two clinical trials demonstrated the safety and effectiveness of the treatment in adults with fibromyalgia.
Rheumatology Influencers: Dr. Dave Has a Passion for Giving Back
Dr. Amish Dave discusses how his volunteer, advocacy & public outreach work stem from his greater commitment to community & helping others.

Rethinking Lupus Nephritis: Are Current Approaches & Guidelines off Target?
A rheumatologist & lupus expert reviews lupus nephritis treatment, questioning proteinuria cutoffs & ACR guidelines, advocating for newer therapies & end-of-treatment biopsies.
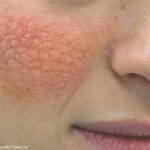
10 Things to Know about Lupus
SLE treatment is rapidly evolving as new therapies are developed & approved. Here are 10 key insights for clinicians.
- 1
- 2
- 3
- …
- 830
- Next Page »