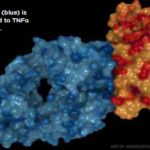
He concluded, “The idea of trying to regulate off-label drug use based on these data seems misguided. If anything, these data support leaving doctors alone and letting doctors continue to make decisions in the best interest in their patients.”
Dr. Joshua M. Sharfstein, from Johns Hopkins Bloomberg School of Public Health, Baltimore, told Reuters Health by email, “The major issue with off-label use is that the FDA has not made a determination as to the balance of risks and benefits.”
ad goes here:advert-1
ADVERTISEMENT
SCROLL TO CONTINUE
The authors reported no funding or disclosures. The two commenters reported advising (unpaid) the FDA.