MADRID—The results of the 52-week, phase 3 RA-BEGIN study were presented at the 2019 European Congress of Rheumatology (EULAR), June 12–15. During the study, researchers assessed the use of baricitinib monotherapy in patients who received it as early treatment for rheumatoid arthritis (RA). They sought to determine if early treatment with baricitinib resulted in improved clinical, functional and radiographic outcomes compared with patients who used methotrexate monotherapy and later switched to baricitinib.1
MADRID—The results of the 52-week, phase 3 RA-BEGIN study were presented at the 2019 European Congress of Rheumatology (EULAR), June 12–15. During the study, researchers assessed the use of baricitinib monotherapy in patients who received it as early treatment for rheumatoid arthritis (RA). They sought to determine if early treatment with baricitinib resulted in improved clinical, functional and radiographic outcomes compared with patients who used methotrexate monotherapy and later switched to baricitinib.1
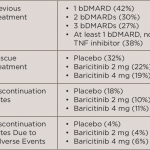
The long-term extension study included 588 patients randomized into three groups to receive either methotrexate, 4 mg of baricitinib daily or combination therapy of methotrexate and 4 mg of baricitinib. Patients who were initially randomized to receive baricitinib monotherapy were defined as the early start group, while methotrexate-treated patients who switched to baricitinib at Week 52 were defined as the delayed start group.
The study assessed the changes from baseline between the two patient groups from Weeks 0–100. Researchers used the Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Disease Activity Score 28-joints–high-sensitivity C-reactive protein (DAS28-hsCRP), Disease Activity Score 28-joints–Erythrocyte Sedimentation Rate (DAS28-ESR), Health Assessment Questionnaire-Disability Index (HAQ-DI) and modified Total Sharp Score (mTSS; Weeks 52–100). The percentage of patients who achieved low disease activity (SDAI score less than 11) and remission (SDAI score less than 3.3) were also assessed.
The Results
Within the first four weeks of switching to baricitinib therapy, the low disease activity and remission response rates of the delayed-start group increased from 60% to 78% and 18% to 31%, respectively. The remission rates reached 47% within Year 1, which were not different from the earlystart group. Similar results were seen for CDAI, DAS28-ESR and DAS28-hsCRP.
Patients in the early-start group had significantly greater changes from baseline in HAQ-DI compared with the delayedstart group. These changes were observed as early as Week 1 and continued up to Week 52. This study showed that, in methotrexate-naive RA patients, improved clinical, functional and radiographic efficacy may be achieved with baricitinib.
After the switch to baricitinib, the delayed-start group had a rapid improvement in HAQ-DI, with similar improvement at four weeks. The rate of structural damage also decreased. Delaying baricitinib initiation for a year led to numerically higher mTSS.
At Week 52 when patients were switched from methotrexate to baricitinib, they experienced a rapid clinical response, leading the majority of patients to achieve similar results as early-start patients within four weeks of the switch. If the therapy goal is to attain rapid and sustained disease activity control, the differences in the HAQ-DI and structural progression shown in this study support an earlier switch to baricitinib in patients who do not obtain disease control with methotrexate monotherapy.
The authors note further analysis may be needed to explore the prognostic factors that will assist clinicians in identifying patients who may benefit from early baricitinib treatment.
Michele B. Kaufman, PharmD, BCGP, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
Reference
- Fleischmann R, Schiff M, Kvien TK, et al. Early vs. delayed start of baricitinib in patients with rheumatoid arthritis in a phase 3 trial of patients naive to methotrexate treatment. Ann Rheum Dis. 2019 Jun;78 (suppl 2):306.