Everybody is talking about biomarkers!
What is a biomarker? According to the NIH Biomarkers Definitions Working Group in 1998, a biomarker is:
A characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathological processes, or pharmacologic responses to a therapeutic intervention.
The ideal biomarker would:
- Be safe and easy to measure;
- Be sensitive and specific for the disease in question;
- Be consistent across gender and ethnic groups;
- Be cost efficient;
- Provide more information than we have now; and
- Be modifiable with treatment.
Source: Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2012 Dec;64(12):1794–1803.
Whether you know it or not, you are already using “biomarkers” every day—blood pressure, lipid levels and glycosylated hemoglobin, to name just a few. In fact, in the treatment of rheumatoid arthritis (RA), you are undoubtedly using erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), and anticitrullinated protein antibodies (ACPA aka anti-CCP). Perhaps you even use Health Assessment Questionnaire (HAQ), RAPID-3 or Disease Activity Score (DAS) in its various iterations (Simplified DAS or SDAS, or Clinical DAS or CDAS).
With respect to RA, we use the biomarkers we have to:
- Identify patients with early RA;
- Predict which patients will have severe disease with radiographic progression (RP);
- Predict which patient will respond to which medications, especially biologic DMARDs;
- Predict who will have toxicity to specific medications; and
- Identify adequate response to medications.
Drawbacks of Current Biomarkers
To some extent, current biomarkers are helpful, but there are significant deficiencies. For example, ESR and CRP are elevated in only 40–50% of RA patients, which limits their application to half our patients. The relationship of CRP to RA disease activity is inconsistent. The Consortium of Rheumatology Researchers of North America (CORRONA) published in 2014 a study of 9,135 patients in their registry, in which they explored the relationship of the Clinical Disease Activity Index (CDAI) to CRP. The CDAI was derived from the more complicated DAS by eliminating the CRP or ESR value, as well as the complex formula for calculation. In patients with high disease activity by CDAI, neither ESR nor CRP was elevated in almost half of patients, and in patients with LOW disease activity, either ESR or CRP was elevated in a third. Therefore, we cannot adequately assess disease activity by the ESR or CRP.
Two prospective studies have shown an association between baseline CRP levels and the subsequent development of erosions by MRI. However, the relationship between CRP and RP is also inconsistent. In one TNF inhibitor (TNFi) trial, CRP was predictive of RP developing in the methotrexate (MTX) plus placebo (PBO) group, but not in the group receiving MTX plus the TNFi.
RF has long been considered predictive of poor outcome. RF has also been used to select patients more likely to respond to rituximab (RTX). However, in one study, the average reduction in DAS28 for the seropositive patients was only 0.35 units greater than in the seropositive patients, a modest increase in benefit.
There is a strong association between ACPA and joint damage. In a 2008 study, the odds ratio for RP was 9.9 for high levels for ACPA, compared to only 2.6 for low/moderate levels. However, this is not consistent across all studies, and it has been estimated that ACPA and other current variables contribute only 32% to the prediction of radiographic progression.
The Path to Better Biomarkers
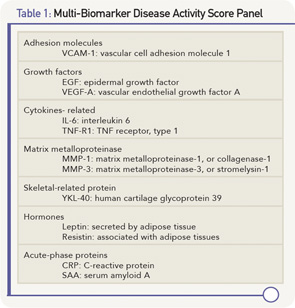
There is general agreement that we need better markers, and there have been many approaches to try to accomplish this. A Multi-Biomarker Disease Activity (MBDA) Score has been developed, starting with almost 400 candidate biomarkers identified by literature review and database screening. One hundred and thirty of these were assayed to identify association with the DAS28CRP and other measures of disease activity and condensed to 25 with the most consistent and strongest association with the DAS28CRP. These were prioritized into the final 12 biomarker algorithm, which is commercially available as Vectra DA from Crescendo Bioscience.
The 12 biomarkers in the MBDA represent multiple pathways that are functionally diverse (see Table 1, above), including cytokines, acute-phase reactant, matrix metalloproteinases (MMP), hormones, growth factors and an adhesion molecule.
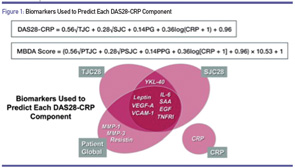
The calculation of the MBDA score involves a formula based on the calculation of the DAS28DA, in which the various components are used to predict the tender and swollen joint counts, and the patient global assessment (see Figure 1). The score is rounded up to the nearest integer on a scale of 0–100. A low score is less than or equal to 29, moderate is 30–44, and high is >44.
Data from the SWEFOT (Swedish Farmacotherapy) trial was analyzed to help assess the clinical usefulness of the MBDA score. SWEFOT enrolled DMARD-naive RA patients with less than one year of symptoms and a DAS28CRP of >3.2. All patients received MTX monotherapy for three to four months, after which they were randomized into two groups. Group A received “triple” therapy with sulfasalazine and hydroxychloroquine added to the MTX; whereas group B added infliximab to the MTX. Each group was followed for a total of 24 months. Attainment of a EULAR “Good” response was no different between the groups: 38% for triple therapy vs. 31% for the infliximab group (p=0.204). However, there was a significant difference in RP, with a mean increase in the van der Heijde modification of the Sharp score of 7.23 in the triple therapy group vs. 4.00 in the infliximab group (p=0.009).
Source: Karsdal MA, Bay-Jensen A-C, Henriksen K, et al. Rheumatoid arthritis: A case for personalized health care? Arthritis Care Res (Hoboken). 2014 Sep;66(9):1273–1280.
Hambardzumyan and colleagues performed a post-hoc analysis of these data to evaluate the MBDA, and found that MBDA at baseline better predicted RP than CRP or DAS28CRP. No patients with a Low score of <29 had RP, whereas 3.4% of the Moderate group (30–44) and 20.9% of the High group had RP. The MBDA helped distinguish those who would vs. would not have RP much better than the ESR, CRP and DAS28CRP. Further, the higher the MBDA score, the greater the likelihood of RP.
This group presented two posters at the 2014 ACR/ARHP Annual Meeting in Boston (RA-Clinical Aspects: Novel Biomarkers and Other Measurements of Disease Activity). They correlated the change in the MBDA from baseline to three months in the SWEFOT data with the likelihood of achieving a EULAR Good response. This actually provided some prediction regarding how patients would do in the two groups. Recall that all patients were on MTX monotherapy during this three-month period. Those who had reduction of >20 points were much more likely to respond to triple therapy (67%) than MTX/infliximab (38%). Conversely, those whose MBDA score improved less than or equal to 20 points were somewhat more likely to respond to infliximab (57%) rather than triple therapy (43%). This is an example of how a biomarker might provide some guidance in terms of whether a patient is more likely to respond to one therapy than another.
Their second poster evaluated the reduction in the MBDA score from baseline to one year as a predictor of RP. Those with high scores (>44) who achieved a reduction to <44 by one year were much less likely to have RP than those whose scores remained >44. Here, a score reduction is correlated with less likelihood of radiographic prediction.
There is a single protein that also has potential as a biomarker for RA, with the unusual name of 14-3-3η. The name refers to chromatography and electrophoresis patterns: elution in the 14th fraction of bovine brain homogenate in DEAE-cellulose chromatography, and location in positions 3.3 of subsequent starch-gel electrophoresis. 14-3-3 is a family of seven intracellular “chaperone” proteins, which fold and move proteins within the cell. The structure includes nine α helices with variable amino and carboxyl termini. The N-terminus forms a cuplike “amphipathic” groove (can bind both hydrophilic and lipophilic structures), which interacts with more than 200 intracellular proteins, representing a wide array of biologic processes: protein synthesis, cellular metabolism, protein trafficking, signal transduction, cytoskeletal transport, apoptosis and transcription.
Further, at the extracellular level, 14-3-3 is a ligand that can stimulate cells of the innate immune system through a variety of transmembrane signaling systems (MAPK/ERK, AKT, JAK-STAT, etc.). This results in nuclear transcription of genes that produce such mediators as MMP-1, MMP-9 and RANKL, leading to joint damage.
Markers of protein degradation & tissue damage that are released into serum are characteristic & reproducible end points of specific pathologic processes.
14-3-3η is the only one of the seven isoforms that is highly upregulated in RA, present in serum and at even higher levels in synovial fluid. The 14-3-3η levels strongly correlate with levels of MMP-1 and MMP-3, and appear to be a marker for more severe disease with radiographic damage. In one study, ESR, CRP, RF, SCPA, HAQ and DAS were all more abnormal in patients who were 14-3-3η positive. Further, positivity and higher titers at baseline correlate with RP five years later. Serial testing shows that a decrease in 14-3-3η correlates with a response to treatment.
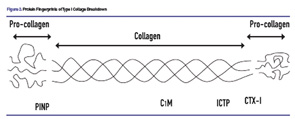
Finally, “protein fingerprinting” can quantitate levels of tissue destruction. Markers of protein degradation and tissue damage that are released into serum are characteristic and reproducible end points of specific pathologic processes. Degradation of Type I collagen offers a good example. There are several “fingerprints” of Type I collagen degradation. C1M is degradation product resulting from MMP-1 activity. ICTP, or C-terminal cross-linking telopeptide of Type I collagen, is also released from connective tissue as a result of MMP activity. CTX-1, or C-terminal telopeptide of Type I collagen, is a marker for bone resorption. By contrast, PINP, or N-propeptide of Type I collagen, is a marker for bone formation. Figure 2 (above) illustrates these protein fingerprints of Type I collagen.
Looking Ahead
Although we do not yet have idea biomarkers for RA, progress has been made. We can hope that over the next few years, we will have even better biomarkers to guide our diagnosis and treatment.
Rick Brasington, MD, is a rheumatologist and fellowship program director at Washington University in St. Louis, and an associate editor of The Rheumatologist.