Methotrexate (MTX) remains the predominant medication used by rheumatologists to treat rheumatoid arthritis (RA). Doses of 7.5–25 mg per week with daily folic acid are generally prescribed. Despite its common use, MTX must be prescribed cautiously given the potential adverse effects when taken incorrectly or without folic acid supplementation.
Cases of MTX-induced cutaneous ulceration have been documented mainly in patients with psoriasis. Below, I report on the case of a patient with seropositive RA who developed cutaneous lesions on her hands and lower legs after incorrect use of MTX (see Image 1, above).
The Case
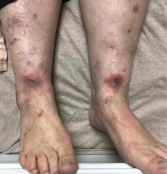
Image 1: Both of the patient’s lower legs and feet featured lesions.
A 65-year-old female with a past medical history of gastroesophageal reflux disease, hypertension and RA (positive tests for anti-cyclic citrullinated peptide [anti-CCP] antibodies and rheumatoid factor) presented at the hospital with a two- to three-week history of sudden-onset abdominal pain, diarrhea, skin rashes and oral ulcers.
She reported that her dose of MTX had been increased from four tablets a week (10 mg) to seven tablets a week (17.5 mg) two months earlier. She had been on MTX for roughly three years and reported compliance with daily folic acid intake. The patient said that when she was on four tablets of MTX weekly, she would take one tablet every other day. Then, when her dose was increased to seven tablets, she took one tablet daily instead of one weekly dose. She had never taken all the tablets at once. Of note, her prescription was actually for six tablets, not seven, to be taken in a single weekly dose.
Her other medications included aspirin daily (81 mg), prednisone daily (5 mg), omeprazole, amlodipine, atorvastatin and valsartan. Of note, the patient did not have a history of psoriasis.
Examination of her skin revealed erythematous indurated plaques with central ulceration and crusting on her lower legs, and similar, smaller lesions on the dorsum of both hands. The patient’s mouth was sore and inflamed, particularly her lower lip and underneath her tongue (stomatitis).
Upon admission, her labs were significant for anemia, with hemoglobin at 9.6 g/dL, and thrombocytopenia, with a platelet count of 76 K/uL. Due to suspicion for MTX toxicity, the primary team gave the patient a 5 mg dose of folinic acid (Leucovorin) orally and started her on folic acid at 1 mg intravenous (IV) daily.
On her second day of admission, the hematology/oncology service evaluated the patient. The physicians believed all of her symptoms were likely related to MTX toxicity and started the patient on 10 mg folinic acid every eight hours for two days. She was also continued on 1 mg of oral folic acid daily.
A dermatologist evaluated her skin lesions, and the differential diagnoses included neutrophilic dermatosis, infection and vasculitis. The dermatologist was less suspicious for an infectious etiology (such as deep fungal or atypical mycobacteria) given how widespread and multifocal the lesions were and the lack of systemic symptoms, such as fever. A punch biopsy was taken and stained with hematoxylin and eosin, and the patient was pan-cultured. Skin biopsy findings revealed cutaneous ulceration due to MTX toxicity.
Over the next three days, the patient’s diarrhea and abdominal pain improved. No new skin or oral lesions developed. Her platelet count started to improve.
Due to symptomatic improvement, patient was discharged after five days in the hospital. She was advised to discontinue MTX and continue daily folic acid at 1 mg daily. Her symptoms continued to improve, and the skin lesions disappeared within two to three weeks of discharge.
Discussion
Low-dose MTX is a rare cause of cutaneous lesions in patients with RA, but a few cases have been reported. At times, cutaneous lesions seen with MTX toxicity can be mistaken for infections and treated with a variety of antibiotics before they are properly identified.1
Methotrexate is a folic acid analog that inhibits the synthesis of DNA, RNA and proteins by binding to dihydrofolate reductase.2 The adverse effects of MTX related to folate antagonism include anemia, neutropenia, stomatitis, oral ulcers and diarrhea due to ulceration/erosion of the gastrointestinal tract. These toxicities can be prevented or alleviated with folate supplementation of 1–5 mg per day. Adverse effects unrelated to folate antagonism include hepatotoxicity, renal insufficiency, pneumonitis, pulmonary fibrosis, fatigue and nodulosis.2
The main cause for the development of adverse effects in our patient was incorrect administration. The patient took 2.5 mg of MTX daily for seven days instead of taking her prescribed dose of six tablets weekly. She did report compliance with taking folic acid 1 mg daily, so that was not a contributing factor.
Upon admission, the patient’s MTX level was checked and was below the level of detection. Because MTX is retained in cells in polyglutamed form, serum blood levels are not good indicators of toxicity. Rescue measures should be undertaken based on clinical symptoms of MTX toxicity and not on the basis of blood levels of the drug.3
Risk factors for development of MTX-induced lesions include new initiation of MTX or reinstatement after hiatus, recent escalation of MTX dose, inappropriate self-medication, renal impairment, age older than 55, folate deficiency, low serum albumin and drug-drug interactions (e.g., NSAIDs, aspirin, trimethoprim/sulfamethoxazole, etc.).3
Of note, our patient was also taking omeprazole and aspirin, and aspirin has been found to decrease MTX clearance by 35%.4 Recent studies have shown the concomitant use of high-dose MTX and proton pump inhibitors may increase MTX levels in the blood and lead to adverse reactions. The findings have mainly been studied with high-dose MTX.5
The histological findings on biopsy were similar to what has been described in literature. The biopsy revealed focal dermal-epidermal separation with keratinocyte dysmaturation and apoptotic keratinocytes, which are due to MTX’s direct toxic anti-metabolite effect on the epidermis.6 The biopsy also revealed irregular acanthosis and perivascular lymphohistiocytic infiltrate, with rarer neutrophils and a few scattered eosinophils. These features can also appear in MTX drug toxicity.3 Although not mentioned in our patient’s biopsy, histology can also reveal a papillary dermis that is edematous with ectatic vessels.3
Upon admission, our patient received 5 mg of oral folinic acid and was started on 1 mg of intravenous folinic acid daily, and on day 2 she was started on 10 mg of oral folinic acid every eight hours for two days. Within three days, the patient’s symptoms had started to improve, and laboratory testing revealed improving pancytopenia. Her rashes continued to heal and resolved within three weeks. Early aggressive therapy with folinic acid likely played a large role in our patient’s quick response to treatment.
In Sum
This case highlights the importance of patient education regarding the administration of methotrexate and its potential side effects if taken incorrectly. A patient’s current medication list should be reviewed in detail to ensure they are not taking medication that could increase the toxicity of MTX by decreasing renal clearance. Finally, rheumatologists should be aware of the appearance of cutaneous lesions due to MTX toxicity, because they may be the first indicator of systemic toxicity.
 Nitasha Kumar, MD, is a rheumatology fellow at Ochsner Medical Center in New Orleans.
Nitasha Kumar, MD, is a rheumatology fellow at Ochsner Medical Center in New Orleans.
Acknowledgments: Dr. Kumar thanks William Davis, MD, and Karen Toribio, MD, attending rheumatologists at Ochsner Medical Center in New Orleans, for their mentorship and assistance with this article.
References
- Lewis HA, Nemer KM, Chibnall RJ, et al. Methotrexate-induced cutaneous ulceration in 3 non-psoriatic patients: Report of a rare side effect. JAAD Case Rep. 2017 Apr 14;3(3):236–239.
- Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(3):168–173.
- Shiver MB, Hall LA, Conner KB, et al. Cutaneous erosions: A herald for impending pancytopenia in methotrexate toxicity. Dermatol Online J. 2014 Jul 15;20(7):5.
- Bookstaver PB, Norris L, Rudisill C, et al. Multiple toxic effects of low-dose methotrexate in a patient treated for psoriasis. Am J Health Syst Pharm. 2008 Nov 15;65(22):2117–2121.
- Suzuki K, Doki K, Homma M, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009 Jan;67(1):44–49.
- Delyon J, Ortonne N, Benayoun E. Low-dose methotrexate-induced skin toxicity: Keratinocyte dystrophy as a histologic marker. J Am Acad Dermatol. 2015 Sep;73(3):484–490.