Polyarteritis nodosa (PAN) is a rare, necrotizing arteritis of the medium or small arteries that lacks the production of anti-neutrophil cytoplasmic antibodies (ANCA).1 PAN has a variety of clinical presentations and a spectrum of severity. Virtually any organ may be involved, and the disease can present with isolated or multisystem involvement. Greater than 90% of all patients experience constitutional symptoms at the time of diagnosis.2 Due to the variable presentation of the disease, the differential diagnosis for PAN is broad and includes infectious diseases, other systemic vasculitides, and vasculopathies, such as segmental arterial mediolysis (SAM) and vascular Ehlers-Danlos (vEDS).3,4
Case Presentations
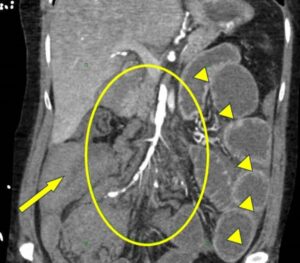
Figure 1. Sagittal section from CTA of the abdomen and pelvis taken on hospital day 12 highlighting SMA involvement in vED. The circle highlights the SMA diffuse irregularity and beading of the arterial wall. Arrowheads show hyperenhancement of the distal small bowel related to intestinal ischemia from distal SMA occlusions. The arrow points to hemoperitoneum. The patient also had dilation of the proximal small bowel with cutoff at the right lower quadrant anastomosis consistent with high-grade small bowel obstruction. (Click to enlarge.)
Case 1
A 38-year-old female with a past medical history of post-traumatic splenectomy, Bernard-Soulier syndrome, uterine rupture and small bowel obstruction presented to the emergency department with epigastric pain, nausea and vomiting. Initial laboratory tests revealed an elevated white blood cell count of 29×103/μL, with a neutrophil predominance, hemoglobin of 11 g/dL and an elevated venous lactic acid of 3.1 mmol/L. Repeat studies over the next several hours noted an acute drop in blood hemoglobin to 4.8 g/dL, with worsening lactic acidosis.
An urgent computed tomography (CT) angiogram of the abdomen showed a moderate hemoperitoneum, right hemothorax and fatty infiltration of the large bowel suggestive of chronic ischemia. Left renal infarcts were noted, as well as a beaded appearance of the celiac, mesenteric and renal arteries, and an aneurysm of the left renal artery (see Figure 1). The findings were reported as concerning for polyarteritis nodosa.
A massive transfusion protocol was initiated, a right chest tube was inserted, and the patient was transferred to the intensive care unit.
Treatment with intravenous methylprednisolone was commenced for suspected vasculitis at a dose of 1 gm daily. The patient continued to require blood transfusions during this time. Interventional radiology and vascular surgery teams were consulted to assist with stabilizing the bleed; however, the patient was deemed at high risk for vessel rupture due to suspected vessel fragility from active inflammation.