“Doctors really like the tool, because it provides a structure for communication in our RA encounters,” she said.
Another project looked at hydroxychloroquine prescribing patterns to see how many patients are prescribed doses generally considered too high, pointing to a need for improved care. “I think that is really the power of RISE—that we can go from data to a quality measurement strategy that’s actually meaningful for us as rheumatologists,” Dr. Yazdany said.
Another project—GRASP or Genome Research in African American Scleroderma Patients—is attempting to use the RISE network to find potential patients to boost enrollment for this important National Institutes of Health-funded study.
“If this pilot [study] is successful, there will be more opportunities like this to use the power of RISE to conduct research that can advance our field,” Dr. Yazdany said.
Gabby Schmajuk, MD, MS, who leads the RISE Data Analytic Center at the University of California, San Francisco (UCSF), said RISE data are uploaded from the practice to the registry each night, with data validation to ensure elements are in the correct fields. Then, the technology vendor does more quality assurance, followed by even more quality review and data cleaning at the data analytic center level. These centers are at UCSF, the University of Alabama, Birmingham, and Duke University, Durham, N.C.
Recently, researchers at UCSF used RISE data to assess prescription patterns among sarcoidosis patients, finding 50% of patients were on a steroid, with 15% on steroid monotherapy and another 15% on prolonged, high-dose steroids.1 Dr. Schmajuk said this indicates the need for disease-modifying agents so patients have choices other than glucocorticoids.
Another RISE project, which is ongoing, involves loading RA clinical data into a deep-learning model to predict flare at a patient’s next visit. Model runs using UCSF data have produced promising results.2
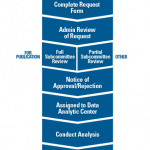
How to Access RISE
Katherine Liao, MD, MPH, chair of the ACR’s Research and Publications Subcommittee to the Research Committee, described a few paths to access to RISE data for research. When investigators want data for publication purposes, they must complete a request form that is reviewed administratively. Next, there’s a full subcommittee review, followed by a notice of approval or rejection. If approved, the project is assigned to a data analytic center.
For requests that are not for publication purposes, such as summary reports, a partial subcommittee review is conducted.