The list of affected drugs includes: carbamazepine (Carbatrol, Equetro, Tegretol, Tegretol XR); clonazepam (Klonopin); clorazepate (Tranxene); divalproex sodium (Depakote, Depakote ER, Depakene); ethosuximide (Zarontin); ethotoin (Peganone); felbamate (Felbatol); gabapentin (Neurontin); lamotrigine (Lamictal); levetiracetam (Keppra, Keppra XR); methsuximide (Celontin); oxcarbazepine (Trileptal); phenytoin (Dilantin Suspension); pregabalin (Lyrica); primidone (Mysoline); tiagabine (Gabitril); topiramate (Topamax); trimethadione (Tridione); valproic acid (Stavzor);and zonisamide (Zonegran).
Many AEDs are used on- or off-label to treat pain syndromes.7 All patients using or starting an AED for any indication should be monitored for notable changes in behavior that could indicate the emergence or worsening of suicidal thoughts, suicidal behavior, or depression. If suicidal thoughts or behaviors emerge during AED treatment, the prescriber should consider whether these symptoms may be related to the illness being treated and act accordingly.
PML with Biologic Therapy in RA
Since the FDA’s removal of efalizumab from the U.S. market, does the rheumatology community still need to worry about PML? In a word, yes. There have been cases of PML, a rare but often fatal opportunistic infection in patients treated with rituximab. The FDA added a boxed warning and updated warning information on the label, noting patients with autoimmune diseases that developed PML had prior or concurrent immunosuppressive therapy. The label also states that PML developed within 12 months of the last rituximab infusion.8
Although natalizumab is not approved for treating rheumatologic conditions, it is used to treat inflammatory bowel disease and multiple sclerosis. With natalizumab in its infancy as a biologic agent for treating immune-mediated diseases, cases of PML began to emerge. The development of these cases prompted the FDA to remove it from the market. It was later re-introduced with prescribing restrictions.9,10
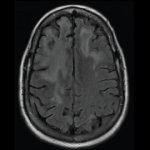
As noted earlier in this article, there has been some concern with MMF and the development of PML. There have been PML cases, sometimes fatal, reported in patients treated with MMF. Because MMF also is used to manage RA patients, it is important to consider PML in the differential diagnosis of a patient that presents with new neurological symptoms, including but not limited to hemiparesis, apathy, confusion, cognitive deficiencies, and ataxia. In the potentially MMF-induced PML cases, patients had PML risk factors, including treatment with immunosuppressive therapies and impaired immune function. In patients presenting with suspect neurologic findings, a consultation with a neurologist should occur as clinically indicated. Physicians should also consider reducing the amount of immunosuppression in patients who develop PML.6
Pipeline
Tocilizumab (Actemra), an anti–interleukin-6 receptor antibody already approved abroad, is pending at the FDA.16 In March, Chugai reported 15 deaths in Japanese patients that used tocilizumab; however, causality was not determined. All of these patients had RA and were treated with tocilizumab. Additionally, interim study results note that in 4,915 patients who have used tocilizumab, pneumonia and severe fever have occurred in more than 200 patients.17