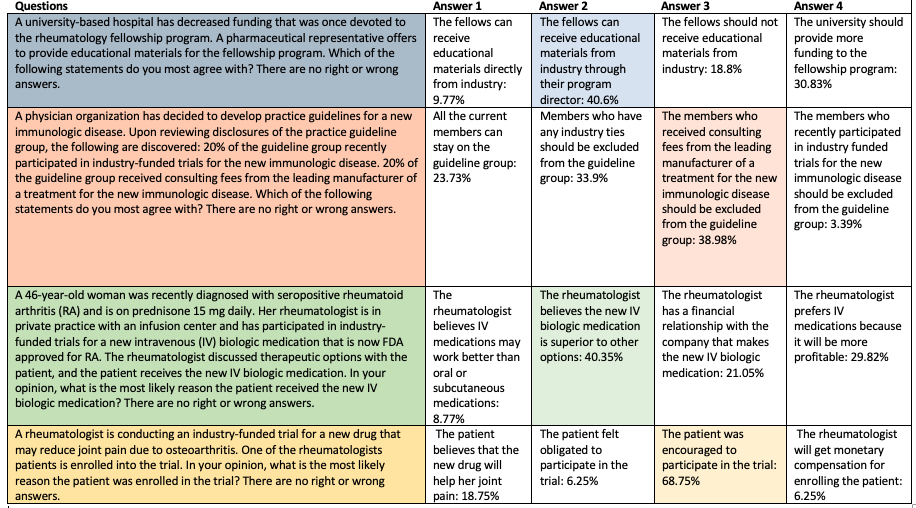
In the final scenario, a rheumatologist is conducting an industry-funded trial on an osteoarthritis drug and a patient is enrolled into the trial. The most popular assessment by far—at 69%—was that the patient was encouraged to participate in the trial, and not that the patient believes the new drug will help her joint pain—a response that was chosen only by about 19% of survey takers.
The survey did not track how employer type—such as academic center or private practice—or other subgroupings responded to the questions.
Dr. Kang says she was intrigued by the split in opinion on the questions—although she was not entirely surprised by it because they are dilemmas that don’t have straightforward answers.
“Those are the situations that are quite complex, and I think that the answers reflect that — where people are divided on how they feel about these ethical dilemmas,” she says.
The ACR’s Position
The ACR requires its volunteers to provide disclosures at the time of nomination and at least yearly so the College can identify, evaluate and manage relationships that may pose “actual, potential or perceived conflicts.” The ACR also requires that everyone in a position to control continuing medical education content disclose all relevant financial relationships with any commercial interest within the past 12 months. Disclosures from the board of directors are posted on the ACR’s website.
Dr. Kang says it’s not adherence to existing policy—which is fairly clear-cut—that poses tough questions routinely for rheumatology providers. It’s the more nuanced situations that are vexing.
“There’s policy, and then there’s what’s really happening,” she says. “In reality, most situations are more gray and there is no clear, right answer. Some people may have very strong opinions. But most people, I think, are not quite sure what to do.”
There is no plan yet on whether and how to incorporate the survey feedback into the ACR’s activities and programming, Dr. Kang says.
“It was an informational, exploratory exercise to understand how people are thinking about these issues,” she says.
Dr. Kang says considering conflict of interest policies and scenarios is particularly important given the explosion of research interest in rheumatology.
“There’s this desire for us as physicians to advance the field, to develop, study and provide new therapies for patients who may not have the treatments that they need—and rheumatology is very exciting because we continue to have a lot of new options for treatment, which really has changed or can change our patients’ lives,” she says. “It just inevitably leads to more ties with industry.