 Baricitinib
Baricitinib
On April 23, the FDA Arthritis Advisory Committee recommended the approval of 2 mg baricitinib for treating adults with moderate to severe active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to methotrexate.1 On the basis of safety and adequacy of risk-benefit profiles, the committee chose not to recommend approval of the treatment at a 4 mg dose.
Baricitinib is a once daily, oral JAK inhibitor. The FDA Arthritis Advisory Committee recommendation was based on four completed phase 3 studies involving a total of 3,492 patients. These studies evaluated RA signs and symptoms, physical function, joint damage progression and patient-reported outcomes. Recognized risks in RA patients were also evaluated, including gastrointestinal perforation, serious infection, major adverse cardiovascular events, malignancy, venous thromboembolism and laboratory function changes.
Baricitinib’s safety profile is based on 7,860 patient-years of exposure.
New Serious Adverse Reaction Identified with Lamotrigine Use
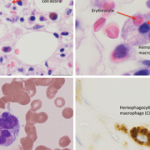
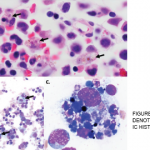
On April 25, the FDA issued a warning that lamotrigine (Lamictal), which is commonly used as an anti-epileptic treatment and for bipolar disorder, that it may cause the rare immune system reaction hemophagocytic lymphohistiocytosis (HLH).2 Patients with HLH most typically present with a persistent and high fever, usually greater than 101°F.
Healthcare professionals must be aware of how to recognize HLH to improve outcomes and decrease mortality. The diagnosis of HLH is often complicated due to its non-specific signs and symptoms, such as fever and rash. Additionally, HLH may be confused with other serious immune-related adverse reactions, such as drug reaction with eosinophilia and systemic symptoms (DRESS).
HLH can occur within days or weeks after beginning therapy. Patients who develop a fever or rash should be promptly evaluated. If HLH or another serious immune-related adverse reaction is suspected and another cause for the patient’s signs and symptoms cannot be identified, lamotrigine should be discontinued.
Patients should also be advised to seek immediate medical attention if they experience HLH symptoms during lamotrigine treatment. Patients or their caregivers should contact their healthcare provider(s) if they experience any HLH symptoms while receiving lamotrigine.
HLH signs and symptoms include unusual bleeding, fever, hepatosplenomegaly, skin or ocular jaundice, skin rashes and swollen lymph nodes. Patients may also experience nervous system problems, including seizures, ataxia, difficulty seeing and/or other visual disturbances. Having at least five of the following eight signs or symptoms is indicative of HLH: fever and rash; hepatosplenomegaly; cytopenias; elevated triglyceride levels or low fibrinogen levels; high blood ferritin levels; hemophagocytosis identified through bone marrow, spleen or lymph node biopsy; decreased or absent natural killer cell activity; elevated blood levels of CD25 showing prolonged immune cell activation.
Information about this serious adverse event with lamotrigine was added as a new warning to the prescribing information.
Michele B. Kaufman, PharmD, BCGP, is a freelance medical writer based in New York City and a pharmacist at New York Presbyterian Lower Manhattan Hospital.
References
- Eli Lilly and Company. New release: FDA Advisory Committee recommends the approval of baricitinib 2 mg, but not 4 mg, for the treatment of moderately to severely active rheumatoid arthritis. 2018 Apr 23.
- U.S. Food and Drug Administration. Drug safety announcement: FDA warns of serious immune system reaction with seizure and mental health medicine lamotrigine (Lamictal). 2018 Apr 25.