References
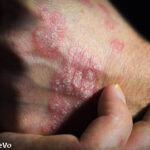
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate to severe plaque psoriasis: Results from a randomized phase 2 study. Br J Dermatol. 2019 Feb 7. doi: 10.1111/bjd.17628. [Epub ahead of print]
- Eli Lilly & Company. A study of mirikizumab (LY3074828) in participants with moderate to severe plaque psoriasis. ClinicalTrials.gov. 2018 Dec 4.
- National Psoriasis Foundation. Mirikizumab (LY3074828). 2019 Mar 19.