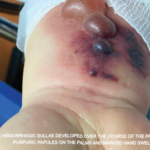
Eosinophilic granulomatosis with polyangiitis (EGPA) is an anti-neutrophil cytoplasmic antibody-associated vasculitis typically characterized by asthma, peripheral eosinophilia and medium- to small-vessel necrotizing vasculitis. Cutaneous manifestations in EGPA are diverse. Palpable purpura is the most common presentation, but urticaria, erythematous macules and papules, livedo reticularis, digital necrosis and cutaneous nodules have also been described.1 Non-hemorrhagic bullae…