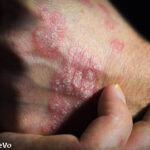
(Reuters)—Drug developer Vitae Pharmaceuticals Inc. said its experimental psoriasis drug significantly reduced the skin condition in patients from a mid-stage trial, sending its shares up 70% in after-hours trading.
Patients taking a 350 mg dose of the drug, VTP-43742, showed a 24% reduction, while patients who took the 700 mg dose showed a 30% reduction compared with placebo.
Psoriasis affects about 7.5 million people in the U.S.
There is currently a range of treatments to improve symptoms, but no cure. The autoimmune disease market is currently dominated by injectable therapies.
VTP-43742, which is a once-a-day oral drug, has the potential to treat other disorders, such as rheumatoid arthritis, multiple sclerosis and inflammatory bowel diseases, the company says.
Psoriasis has been targeted as a promising treatment area by a number of drugmakers, including Novartis AG, which recently launched a product called Cosentyx (secukinumab) for the condition.
This month, U.S. drugmaker AbbVie Inc. bought marketing rights to a promising experimental psoriasis treatment from Germany’s Boehringer Ingelheim for an initial upfront payment of $595 million.