In the previous year, her creatinine increased steadily from 1.2 to 2.3 mg/dL (normal range 0.50–1.20 mg/dL) at the time of admission. Additionally, nephrotic range proteinuria was noted (urine protein/creatinine ratio = 3,230 mg/g). Immunology workup was positive for ANA (homogeneous pattern, titer of 1:320), anti-histone antibodies (moderate 1.6; normal range <1 negative), CCP (>250 units, normal range <20 units), rheumatoid factor (506 IU/mL; normal range <14 IU/mL) and SSA/SSB >8 antibodies (normal range; negative).
In contrast, cryoglobulins, dsDNA, Sm/RNP, ANCA (PR-3, MPO), anticardiolipin, anti-B2-glycoprotein antibodies and lupus anticoagulant screens were negative. The infectious workup included HIV and hepatitis A, B and C tests that were also negative.
An anemia workup was suggestive of anemia of chronic disease. Further testing included serum electrophoresis that did not reveal any monoclonal spike. Interestingly, the subtypes of IgG found a mildly decreased serum IgG1 (333 mg/dL; normal range 382–929 mg/dL). Both C3 and C4 serum complements were significantly reduced (C3 57 mg/dL; normal range 83–193 mg/dL; C4 -4 mg/dL; normal range 15–57 mg/dL).
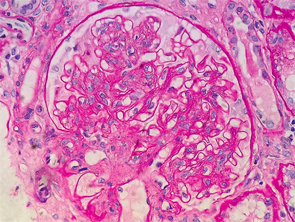
Figure 1: Renal biopsy. H&E staining: enlarged glomeruli, mesangial expansion with diffuse increased cellularity; glomerular basement membranes irregularly thickened, but no vasculitis, necrotizing lesions, crescents or hyaline thrombi were seen within the capillary loops.
Creatinine worsening accompanied by nephrotic range proteinuria, low serum complements levels, positive ANA, SSA/SSB and anti-histone antibodies in the context of active RA disease suggested an autoimmune process as a plausible cause of current renal failure.
Initial fluid resuscitation resulted in no improvement of the patient’s renal function. The decision to pursue renal biopsy was made after consultation with the nephrology service.
Interestingly, the renal biopsy was consistent with immune complex-mediated MPGN. Pathology reported enlarged glomeruli, mesangial expansion with diffuse increased cellularity; the glomerular basement membranes were irregularly thickened, but no vasculitis, necrotizing lesions, crescents or hyaline thrombi were seen within the capillary loops (see Figure 1).
Immunofluorescence staining revealed predominant IgG and C3 deposition in the glomerular basement membrane and mesangial compartment. Additionally, with lower predominance the immunostaining was positive for IgM and IgA deposition. However, immunostaining was negative for C1q or hyaline thrombi usually seen in lupus nephritis and renal vasculitis, respectively. Electron microscopy confirmed the irregular thickening of the glomerular basement membrane, with a few scattered subendothelial electron dense deposits, focal neomembrane formation, an expanded mesangium and podocyte foot processes effacement (see Figure 2).
The patient was treated with high-dose pulse steroids for three days (methylprednisolone 1 g/day), followed by prednisone 1 mg/kg/day. Hydralazine was discontinued shortly after admission and replaced with amlodipine. While in the hospital, she received the first dose of Rituximab (1,000 mg), followed by a second dose 14 days later. On Day 7 of admission, the patient’s complaints subsided, and her creatinine was noted to decrease to 1.55 mg/dL.

