Pipeline and Drug Approvals
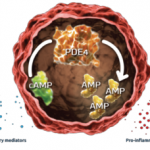
A new drug application (NDA) has been submitted to the Food and Drug Administration (FDA) for apremilast (CC-10004) to treat psoriatic arthritis.1 An FDA response is expected sometime this month. Apremilast is an oral phosphodiesterase 4 (PDE4) inhibitor that regulates inflammatory and immune processes.2 It has been shown to quell a number of different cytokines (e.g., tumor necrosis factor–alfa, interleukin [IL-] 12, IL-23, and interferon-gamma) and chemokines, improving a patient’s inflammatory response. The manufacturer of apremilast has also submitted an NDA for the drug to treat psoriasis.3 Rheumatoid arthritis (RA) and ankylosing spondylitis (AS) trials are currently in phase III. Results of the psoriatic arthritis trials were recently presented at the ACR/ARHP Annual Meeting in San Diego. The first phase III trials (PALACE 1, 2, and 3) compared apremilast to placebo in patients who had previously been treated with biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs). In the phase III “Efficacy and Safety Study of Apremilast to Treat Active Psoriatic Arthritis (PALACE 2)” trial, patients were allowed to continue DMARDs, including sulfasalazine, leflunomide, methotrexate (MTX), or a combination of these.4 There were 159 patients randomized to received placebo, 163 patients in the 20-mg twice-daily (BID) apremilast treatment arm, and 162 patients in the 30-mg BID apremilast treatment arm. After Week 16, placebo-treated patients were randomized to an active treatment group. Following one year of treatment, the Psoriasis Area and Severity Index (PASI) 50/PASI 75 was achieved by 49% of the 20-mg BID–treated patients and 59% of the 30-mg BID–treated patients. Adverse reactions were mild to moderate, and most patients did not stop treatment due to these adverse reactions. Adverse reactions occurred in 5% of patients and included nausea, diarrhea, and headache. PALACE 4 utilized apremilast 20 mg BID for a 16-week treatment duration. Twenty-nine percent of patients achieved an ACR 20 response.1 In patients who received 30 mg BID, 32% achieved an ACR 20 response, and placebo-treated patients had a 17% response. After Week 52 of treatment, 53% of the 20-mg BID–treated group achieved an ACR 20, and 59% of the 30-mg BID–treated group achieved an ACR 20. In addition, 14% and 18%, respectively, achieved an ACR 70 response at Week 52.
Diclofenac sodium topical solution (Pennsaid 2%) has been FDA approved as a BID nonsteroidal antiinflammatory drug (NSAID) to treat pain associated with osteoarthritis (OA) of the knee.5 This formulation carries the same risks as other oral and topical NSAIDs, including cardiovascular and gastrointestinal risks and liver function test (LFT) elevations. This is a follow-on product to the diclofenac sodium 1.5% topical solution product. It has a higher strength than the former product and is more viscous.
The FDA has accepted an NDA for methotrexate (MPI-2505), a subcutaneous (SubQ) MTX in a ready-to-use “autopen” injection device, available for drug concentrations of 30 mg/mL or more.6 The proposed indications for this device are RA, polyarticular-course juvenile RA, and psoriasis.
Drug Safety Antidepressant Hepatotoxicity
Drug-induced liver injury (DILI) is a major cause of liver damage, accounting for more than 50% of cases of acute liver failure.7 Recently, Voican et al published their review for clinicians on this topic. They conducted a PubMed search evaluating the potential for DILI related to antidepressants, evaluating clinical data on antidepressant-induced liver injury from 158 reports, including 88 case reports, 38 original articles, and 32 reviews dating back to 1965.8 They noted that data on antidepressant liver injury is scarce, but calculated that 0.5% to 3% of patients treated with antidepressants may develop asymptomatic mild serum alanine aminotransferase (ALT) level elevations. DILI is more common in patients taking multiple medications and in the elderly. Additionally, the risk of DILI may also increase with concomitant use of one or more agents that target the same cytochrome P450 (CYP450) isoenzyme pathway. Antidepressant-induced liver injury is usually considered to be dose independent; however, dose escalations of duloxetine, nefazodone (no longer available in the United States), sertraline, and mianserin (a tetracyclic antidepressant not available in the United States, but similar to mirtazapine) have led to toxicity in some case reports. Hepatotoxicity is usually idiosyncratic and unpredictable. In a retrospective analysis of DILI cases reported in the U.S., daily drug doses of ≥50 mg were associated with a higher risk of liver failure. Based on the evidence, the highest-risk antidepressants are the monoamine oxidase inhibitor class, and nefazodone, imipramine, amitriptyline, duloxetine, bupropion, trazodone, tianeptine (a selective serotonin reuptake enhancer not available in the U.S.), and agomelatine (a melatonergic antidepressant not available in the U.S.). Agents that appear to have a lower risk of DILI include citalopram, escitalopram, fluvoxamine, and paroxetine. Liver injury usually occurs within six months of beginning treatment. Although infrequent, antidepressant DILI may be irreversible and life threatening. Clinicians need to be aware of this because patients are often treated with concomitant potential hepatotoxins or agents that target the same CYP450 isoenzyme pathway. Monitoring for serum ALT level elevations is the best surveillance to detect potential DILI. Prompt drug discontinuation is critical.
Dr. Kaufman is a freelance medical writer based in New York City, a pharmacist at New York Presbyterian–Lower Manhattan Hospital, a certified geriatric pharmacist, and adjunct faculty at Touro College of Pharmacy.
References
- Taylor P. Celgene lifted by apremilast data in psoriatic arthritis. Published October 28, 2013. www.pmlive.com/pharma_news/celgene_lifted_by_apremilast_data_in_psoriatic_arthritis_513370#. Last Accessed January 27, 2014.
- Palfreeman AC, McNamee KE, McCann FE. New developments in the management of psoriasis and psoriatic arthritis: A focus on apremilast. Drug Des Devel Ther. 2013;7:201-210.
- Celgene product pipeline. Published January 6, 2014. https://www.celgene.com/research-development/product-pipeline. Last Accessed February 2, 2014.
- Susman E. Apremilast works in psoriatic arthritis. Published October 30, 2013. www.medpagetoday.com/MeetingCoverage/ACR/42568. Last Accessed January 27, 2014.
- Nuvo research announces DA approval of PENNSAID 2%. Published January 17, 2014. www.firstwordpharma.com/node/1179866#axzz2sCFQ7HiI. Last Accessed January 20, 2014.
- Medac Pharma Inc secures FDA acceptance of a New Drug Application (NDA) for Methotrexate-Containing Autopen. Published January 27, 2014. www.medacpharma.com/medac-pharma-inc-secures-fda-acceptance-of-a-new-drug-application-nda-for-methotrexate-containing-autopen. Last Accessed January 29, 2014.
- Nathwani RA, Kaplowitz N. Drug Hepatotoxicty. Clin Liver Dis. 2006;10:207-217.
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: A review for clinicians. Am J Psychiatry. 2013 Dec 20. [Epub ahead of print]