Have you recently asked a patient about his or her ability to smell? Can you recall a patient complaining about losing his or her sense of smell? Have you ever used this complaint to determine the patient’s diagnosis?
Surprisingly, an impairment in smell may be an important manifestation in systemic lupus erythematosus (SLE). Indeed, the sense of smell may be a clue to what is going wrong in the patient’s nervous system, pointing to a new line of brain research to understand pathogenesis. In this article, I will describe how I became interested in smell and why I think the nose is another organ to consider in understanding this complex multisystem disease.
SLE is a disease of immune disturbance characterized by over 156 different autoantibodies.1 In antiphospholipid syndrome (APS), serological analysis can demonstrate the presence of more than 40 different autoantibodies in the blood.2 Furthermore, in reviewing the literature, my colleagues and I have found that central nervous system (CNS) involvement in SLE and APS is associated with close to 20 different specific autoantibodies.3 One that stands out prominently is the anti–P-ribosomal (anti-P-R) antibody. My interest in the sense of smell arose incidentally when we analyzed the potential pathogenic role of anti-P-R autoantibodies in CNS involvement of SLE.4-6
For years, the association of anti-P-R antibodies with CNS involvement of SLE has been reported in the literature. The role of anti-P-R antibodies in this manifestation was highlighted by the remarkable report by Bonfa and colleagues.7 They demonstrated a clear correlation between highly elevated titers of anti-P-R antibodies and psychotic events in SLE patients. This association strongly suggested that autoantibodies may cause psychiatric manifestations. The role of immunological factors in CNS manifestations in SLE gained further support from the observations that, when the autoimmune phenomena of SLE are treated with drugs such as corticosteroids or other immunosuppressives, the psychiatric findings and symptoms (e.g., cognitive impairment, major depression or psychosis, etc.) can subside. While speculative, these findings may relate to the broader issue of whether “idiopathic” psychiatric diseases such as schizophrenia and manic-depressive states may also have, in part, an autoimmune origin.8,9


Early Research in Mice
My colleagues and I collaborated with Morris Reichlin, MD, professor of medicine at the University of Oklahoma Health Sciences Center in Oklahoma City, and his colleagues to test in an in vivo model the effects of anti-P-R antibodies on brain function. We injected anti-P-R antibodies purified by affinity chromatography from the serum of a depressive SLE patient directly into the ventricles of the brains of naive mice. We wanted to avoid the blood–brain barrier, an obstacle that could also be overcome by injecting the autoantibody intravenously either together with epinephrine, imitating a state of stress, or with lipopolysaccharide, mimicking a state of infection.10
The intra–cerebro-ventricular injected mice were analyzed by a variety of established, well-validated neurological tests. To our surprise, in contrast to the control injected mice (who received irrelevant immunoglobulins), the anti-P-R antibody–treated mice developed defects in cognition and a severe depressive behavior. In a standard method to assess depression, mice are placed into a bucket of water. While normal mice will swim endlessly in the bucket to rescue themselves, depressed mice will give up and just float (see Figure 1, p. 17). In this model, mice injected with anti-P-R antibodies floated, indicating that they were depressed.10
As shown subsequently in immunohistochemistry experiments, the anti-P-R antibodies bound specifically to the limbic areas of the mice brains and amygdala.10 Moreover, the anti-P-R antibodies were able to bind to the hippocampal cells as well as to penetrate their cytoplasm. A similar pattern of binding and behavior was also shown by us later on, also for the anti-DNA monoclonal antibodies carrying the pathogenic 16/6 idiotype.11
Because the limbic areas of the brain are involved in smelling capabilities, we analyzed the mice subjected to the anti-P-R and anti-DNA autoantibodies (16/6+) and found a lower capability to smell (determined by studying the mice exposed to obnoxious odors) (see Figure 2, p. 17). After resection of the olfactory bulb, mice develop a depressive pattern of behavior that can be overcome by fluoxetine (Prozac). Similarly, fluoxetine can shorten the time of floating of mice injected with anti-P-R antibodies, indicating that depressive behavior was reduced.

An important breakthrough came when we investigated the influence of fragrance on mouse behavior. We found that exposing the mice to an orange fragrance increased their mobility time while swimming in the bucket, an idea we had after reading several papers on experimental models showing that lemon and citrus exposure of rats normalized neuroendocrine levels and immune function and were rather more effective than anti-depressants.12 Similar results were achieved by lavender fragrance, which also had a beneficial effect on insomnia and depression in female college students.13
The interbrain connections between specific smells have yet to be analyzed for their mechanisms, and the results of such study may lead to novel anti-depressant agents.
On to Human Trials
Given the observations in mice, my colleagues and I wanted to see whether patients with SLE also had an olfactory abnormality. We performed the test of smell, a simple evaluation that uses 48 smell sticks. The person examined is first trained to recognize the smells, and is then exposed to sticks that also contain “control” smells. The ability to recognize smell is absolute as well as “dose” dependent.
The patients with SLE that we examined using the smell test fared significantly worse than age- and sex-adjusted controls. The impaired smelling capacity correlated with the SLEDAI activity index and was more prominent among patients with CNS involvement.14 These results are in accord with recent anatomical observations, including MRI scans, showing that patients with SLE have a reduction in the size of their amygdala that correlates with the duration and activity of the disease.15,16
Being a clinician and a basic immunologist, I was very excited by each discovery. Yet, being skeptical, I was always surprised to see how much is known and already published in the literature, but ignored by the conservative physician world. I shared my findings with my neighbor (a “natural” healer). She smiled at me saying, “We’ve know this for years! And we’ve used it for years!”
I will note that aromatherapy was far from my interest but, considering our results, I started to dwell into this field of complementary and alternative medicine. Like all other fields of alternative medicine, aromatherapy lacks many well-controlled, double-blind studies, although a few such studies exist (cited herein) and, in my research, we are dealing with chemicals (fragrance) and neuronal receptors. However, it seems that a more intensive research of the effects of lupus on olfaction may lead to the better understanding of neural disturbance in this disease and the development of new series of therapies that may involve using aromatherapy to improve mood.
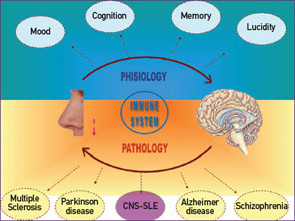
Defects in smelling capabilities have already been reported in other autoimmune diseases such as multiple sclerosis and in other neurological diseases conditions including Parkinson’s and Alzheimer’s diseases.17-19 (See Figure 3, above.) The dampened smelling capability appears at the early stages of these diseases and may have a predictive value for ultimate outcome. Aberrant smell function also characterizes depressive women and can be ameliorated by exposing the patients to orange or lavender fragrances (i.e., aromatherapy).
The 2004 Nobel Prize in Medicine was granted to Linda Buck and Richard Axel for research on odorant receptors and olfactory system organization, and for deciphering the 1,000 genes for odor (in humans, only 350 genes still function). This seminal research emphasized the diversity of smell options (especially if combined with memory and emotions) in comparison to other senses, such as taste or vision, which only have a few associated genes.
In summary, it seems that as clinicians, we might do well to return to basics by either asking our patients about their sense of smell or by performing a simple physical diagnostic test to evaluate the function of this sense. Defects in smell function may indicate a specific CNS involvement in SLE and other autoimmune rheumatic diseases, as well as other common neurological diseases. Moreover, developing specific fragrances already known to the ancient Egyptians and Greeks may add another tool to treat hitherto unexplained psychiatric conditions.
Last—but not least—our induction of pure psychiatric states using autoantibodies may relate to the possibility that some of the cases of idiopathic psychiatric states have an autoimmune origin.8
Dr. Shoenfeld is professor in the department of medicine ‘B’ and Centre for Autoimmune Diseases at Sheba Medical Centre in Tel-Hashome and incumbent of the Laura Schwarz-Kipp Chair for Research of Autoimmune Diseases in the Sackler Faculty of Medicine at Tel-Aviv University, both in Israel.
References
- Sherer Y, Gorstein A, Fritzler MJ, Shoenfeld Y. Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. Sem Arth Rheum. 2004;34:501-537.
- Shoenfeld Y, Twig G, Katz U, Sherer Y. Autobntibody explosion in antiphospholipid syndrome. J Autoimmun. 2008;30:74-83.
- Zandman-Goddard G, Chapman J, Shoenfeld Y. Autoantibodies involved in neuropsychiatric SLE and antiphospholipid syndrome. Semin Arthritis Rheum. 2007; 36:297-315.
- Mahler M, Kessenbrock K, Szmyrka M, et al. International multicenter evaluation of autoantibodies to ribosomal P proteins. Clinical and Vaccine Immunol. 2006;13:77–83.
- Toubi E, Shoenfeld Y. Clinical and biological aspects of anti-P-ribosomal protein autoantibodies. Autoimmunity Rev. 2007;6;119-125.
- Shoenfeld Y. The diversity of autoantibodies to P-ribosomal: The infectious-autoimmunity plot. J Mol Med. 2007;85:907-909.
- Bonfa E, Golombek SJ, Kaufman LD, et al. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265-71.
- Teplizki-Amital H, Sela B, Shoenfeld Y. Autoantibodies to brain pulynucleotides in patients with schizophrenia. Immunol Res. 1992;11:66-73.
- Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: A comprehensive model updated and revisited. J Autoimmunity. 2006;27:71-80.
- Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56:938-948.
- Blank M, Shoenfeld Y. The story of the 16/6 idiotype and systemic lupus erythematosus. IMAJ. 2008;10:37-39.
- Komori T, Fujiwara R, Tanida M, Nomura J, Yokoyama MM. Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation. 1995;2:174-180.
- Lee IS, Lee GJ. [Effects of lavender aromatherapy on insomnia and depression in women college students] [Article in Korean]. Taehan Kanho Hakhoe Chi. 2006;36:136-143.
- Ben-Ziv T, Katzav A , Chapman J, Blank M, Reichlin M, Shoenfeld Y. The smell of autoimmune depression: Anti-ribosomal P antibodies induce depression and impaired olfactory function journal of autoimmunity. 2009, in press.
- Emmer BJ, van der Ground J, Steup-Beekman GM, Huizinga TW, van Buchem MA. Selective involvement of the amygdala in systemic lupus erythematosus. PloS Med. 2006;3: e498.
- Appenzeller S, Carnevalle AD, Li LM, Costallat LTL, Cendes F. Hippocampal atrophy in systemic lupus erythematosus. Ann Rheum Dis. 2006;65:1585-1589.
- Shoenfeld Y. To smell autoimmunity: Anti-P-ribosomal autoantibodies, depression, and the olfactory system. J Autoimmunity. 2007;28:165-169.
- Pause BM, Miranda A, Göder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271-277.
- Strous RD, Shoenfeld Y. To smell the immune system: Olfaction, autoimmunity and brain involvement. Autoimmune Rev. 2006;6:54-60.
- Stojanovich L, Zandman-Gosddard G, Pavlovich S, Sikanich N. Psychiatric manifestations in systemic lupus erythematosus. Autoimmunity Rev. 2007;6:421-426.
- Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505-2514.
- Diamond B, Kowal C, Heurta PT, et al. Immunity and acquired alterations in cognition and emotion: Lessons from SLE. Adv Immunol. 2006;89:289-320.
- Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity: antibody impairs memory. Immunity. 2004;21:179-188.