up visits occurring every two weeks. Follow-up visits included adverse event assessment, concomitant medication update, blood and urine sample collection, and a physical exam.
The primary outcome was the proportion of responders—patients who achieved an sUA of less than 6 mg/dL for more than 80% of the time at six months.
Examining the Results
Seventeen patients were screened for study inclusion, and 14 ultimately received at least one dose of pegloticase. Eleven patients completed pegloticase and methotrexate treatment for 24 weeks. All patients were men, with a mean age of 49.3 years; 11 of the 14 patients were white.
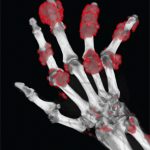
On day 1, the mean sUA was 9.2 mg/dL; 12 of 14 patients had visible tophi.
At six months, 11 patients (78.6%) were responders, with three patients meeting stopping criteria. For these three patients, loss of response occurred after two infusions, three infusions and five infusions (one patient each).
The mean total methotrexate dosage was 64.4 mg during the methotrexate run-in period, with no dosage reduction occurring.
Before therapy with pegloticase and methotrexate, patients had a mean of 6.4 joints affected by tophi. By the last assessment, the number had been reduced to a mean of 2.6 joints.
The adverse events seen most frequently were gout flare, nausea and abdominal discomfort during the run-in period. During the co-treatment, all patients had at least one adverse event, including gout flare (85.7%; the flares were mild to moderate in most patients), diarrhea (21.4%) and upper respiratory tract infection (21.4%). There were no major adverse cardiovascular events.
Thirteen of 14 patients had stable or improved kidney function. No increases occurred in liver function tests.
Clinical Implications
The results of the MIRROR study demonstrate the need for a controlled trial to help confirm the findings are not confounded by differences in steroid prophylactic agents, according to the study researchers. In fact, such a trial is currently underway with the MIRROR RCT, according to according to John K. Botson, MD, a rheumatologist with Orthopedic Physicians Alaska in Anchorage, and the lead author of the MIRROR open-label study.
Topline results from MIRROR RCT were reported in October 2021: Primary endpoints were met, and 71% of patients with uncontrolled gout achieved a complete response rate using pegloticase with immunomodulator methotrexate.4

Dr. Botson
Dr. Botson is also involved with other research in this area, including the AGILE trial to evaluate a shorter infusion duration for pegloticase prescribed with methotrexate; the FORWARD trial to evaluate pegloticase dosing options given every four weeks; and the ADVANCE trial to evaluate pegloticase with methotrexate to treat those with previously developed anti-drug antibodies on pegloticase monotherapy.
Michael Toprover, MD, rheumatologist and assistant professor of medicine at NYU Langone Health in New York City, says the MIRROR open-label study is an important stepping stone to support adding an immunomodulator to decrease the risk of anti-pegloticase antibody formation and help patients. He is curious to see the results of a larger trial, particularly with the risks associated with immunomodulatory therapies.
“More robust data need to be collected on the overall safety of these medications in gout patients,” Dr. Toprover says.
He would like to see the results from a randomized trial fall in line with the open-label study and find out how the co-treatment fares in Black and Hispanic patients, to ensure that any effects are generalizable beyond white patients.
An improved response from pegloticase with an immunomodulator, such as methotrexate, can help patients who are struggling with disability and a decrease in their quality of life. “For these patients, pegloticase is the last line of treatment for a painful and damaging chronic disease,” Dr. Botson says. “The better we can make this medication work, the better it’s going to be for our patients.”
Vanessa Caceres is a medical writer in Bradenton, Fla.
References
- Botson JK, Tesser JRP, Bennett R, et al. Pegloticase in combination with methotrexate in patients with uncontrolled gout: A multicenter, open-label study (MIRROR). J Rheumatol. 2021 May;48(5):767–774.
- Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: Two randomized controlled trials. JAMA. 2011 Aug 17;306(7):711–720.
- Keenan RT, Baraf HSB, LaMoreaux B. Use of pre-infusion serum uric acid levels as a biomarker for infusion reaction risk in patients on pegloticase. Rheumatol Ther. 2019 Jun;