ellepigrafica / shutterstock.com
In an international clinical trial, adding the drug belimumab to standard maintenance therapy for patients in remission with vasculitis did not lower the relapse rate. The double-blind, placebo-controlled study evaluated the safety and efficacy of belimumab as adjunctive therapy to maintain remission in anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV). Results of the multi-center, industry-sponsored study were published recently in the journal Arthritis & Rheumatology.1
“There was no evidence that patients on belimumab had fewer or more relapses than those patients on placebo,” says Peter Merkel, MD, corresponding author for the study and professor of medicine and epidemiology, and chief of rheumatology, at the University of Pennsylvania, Philadelphia.
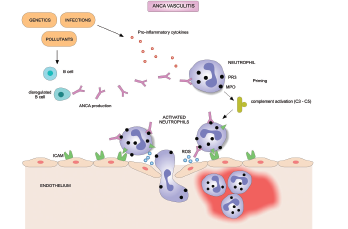
Treatment of ANCA-associated vasculitis often leads to remission, but a high relapse rate is associated with the disease. A major clinical problem in AAV is a need for better treatment regimens to stop the disease from returning once a patient achieves remission, according to study authors.
“After induction of remission, without continued therapy and sometimes even with therapy, the relapse rate can be quite high,” says Dr. Merkel. “Relapse brings about the potential for the same type of dangerous manifestations that initial presentation does, and the cumulative burden of recurrent disease in AAV can be substantial for patients both through the damage induced by the disease itself, and the toxicity of the medications.”
A protein called the B lymphocyte stimulator (BLyS) associated with autoantibody production has been implicated in the pathogenesis of AAV. Elevated levels of BLyS have been observed in patients with AAV and in lupus patients following rituximab treatment, according to the article.
The drug belimumab is a human monoclonal antibody that inhibits BLyS and is approved to treat systemic lupus erythematosus, says Dr. Merkel. The drug has a well-known safety profile and was tested in a clinical trial because high BLyS levels seem to be part of the pathogenesis of ANCA-associated vasculitis.
“The goal was to see if belimumab was helpful to prevent relapse,” says Dr. Merkel, who notes that standard therapy to maintain remission includes a low dose of glucocorticoids plus another immunosuppressive drug such as azathioprine methotrexate or rituximab. “The hypothesis tested in the trial was that belimumab could be an additive and efficacious therapy for maintenance of remission.”
The Study

Dr. Merkel
Researchers recruited patients from 15 countries to participate in the study conducted in 37 centers located in North and South America, Europe and Australia. The pharmaceutical company GlaxoSmithKline sponsored the trial.
Patients were included in the study if they were in remission after induction therapy and had relapsing granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA), two subtypes of AAV characterized by the presence of autoantibodies. Both disorders cause life-threatening blood vessel inflammation that can affect the lungs, kidneys, respiratory and gastrointestinal tracts, skin, heart and nervous system.
Among 105 patients in the study, 53 were randomized to receive belimumab and 52 to receive placebo. All patients who received treatment were in remission at baseline and had received induction therapy in a combination of glucocorticoids and either cyclophosphamide (78 patients) or rituximab (27 patients).
More patients had GPA (83) than MPA (22) in both the placebo and belimumab groups. Overall, patient demographics were similar across treatment groups, but more patients were 65 or older in the belimumab group (18) than in the placebo group (8), according to the article.
The primary endpoint was time to first protocol-specified event (PSE), which was defined as a Birmingham Vasculitis Activity Score (BVAS) of six or greater, the presence of one or more major BVAS item, or receipt of prohibited medication that resulted in treatment failure. Researchers defined vasculitis relapse as the PSE of either a BVAS activity score of six or greater or vasculitis medications that were prohibited in the study.
Results showed using belimumab as an add-on to maintenance treatment did not reduce the risk of PSE or vasculitis when compared with placebo. The difference in time to first PSE was not statistically significant between the belimumab and placebo groups, according to the article.
The overall PSEs rate was low in both groups: 10 out of the 53 patients (18.9%) who received belimumab, and 11 out of 52 patients (21.2%) who received placebo. Vasculitis relapses occurred in six patients (11.3%) in the belimumab group and eight patients (15.4%) in the placebo group.
All vasculitis relapses in the belimumab group were among patients who had proteinase 3 (PR3) ANCA-associated vasculitis with cyclophosphamide-induced disease remission. None of the patients from the belimumab group who had received rituximab induction therapy suffered relapses, a development the authors suggest deserves further study.
For the placebo group, relapses occurred independent of the induction therapy type, disease stage or ANCA type, according to the article.
Adverse events were as expected, says Dr. Merkel, and were recorded in 49 out of 53 belimumab patients and 43 out of 52 placebo patients. None posed any new safety concerns, according to the article.
The goals were revised during the study, which spanned 2013–2017, and the patient sample size was reduced from 300 because of prevailing use of rituximab not only for induction therapy, but also to prevent AAV relapse, notes Dr. Merkel.
“Recruitment was a challenge because of the [increasing] use of rituximab for maintenance of remission during this trial due to changing practice patterns, increasing data on the benefits of rituximab and increasing approval and availability of rituximab worldwide,” says Dr. Merkel.
It was “intriguing” to see that none of the patients who received both rituximab and belimumab had a relapse, even though the sample size was not enough to be definitive, says Dr. Merkel. In conclusion, the combination of the drugs tested appeared safe, the study provided useful information and it demonstrates the ability of the international vasculitis research community to conduct randomized trials in this rare disease, he says.
“The trial does not support the use of belimumab in ANCA-associated vasculitis to maintain remission,” says Dr. Merkel “However, that does not necessarily mean that BLyS is not part of the pathophysiology, nor does it mean that belimumab or a similar agent might not have a role in this treatment.”