T-Cell Signaling Abnormalities: Gene Expression
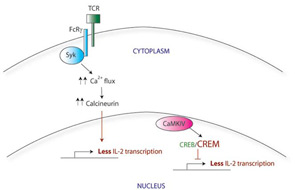
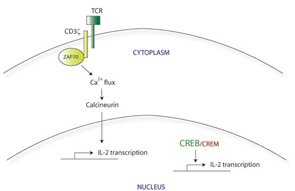
There appear to be many explanations for why T cells from SLE patients express lower levels of CD3ζ. These include decreased transcription, decreased mRNA stability, alternative splicing, and increased protein degradation. For example, the CD3ζ gene promoter contains a cyclic AMP response element (CRE). The CRE-modulator (CREM) transcription factor binds to this element, repressing CD3ζ transcription. The IL-2 gene promoter also contains a CRE site; CREM represses IL-2 transcription in a similar manner. In contrast, the CRE-binding protein (CREB) also binds to the same CRE site, but acts as an activator of IL-2 transcription. SLE T cells show simultaneously increased CREM expression and decreased CREB activity.4 Taking together data from these experiments, it appears that this CREB/CREM imbalance in SLE T cells at least partially explains the decreased CD3ζ and IL-2 expression in these cells.
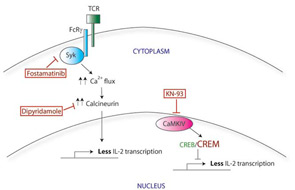
Correcting the CREB/CREM imbalance (and by translation, the defect in CD3ζ and IL-2) opens up a new area for therapeutic intervention. CREM expression is regulated in part by a calcium-responsive kinase, the calmodulin-dependent kinase IV (CaMK IV), and SLE patients show higher levels of CaMK IV in the nucleus. Interestingly, KN-93, a small molecule inhibitor of CaMK IV, has shown efficacy in treating mouse models of lupus, suggesting a promising area for investigation of new therapy.5
Functional Abnormalities of T Cells in SLE
Similar to the situation with T-cell signaling, studies have identified a variety of defects in T-cell function in SLE. For example, cytotoxic activity of CD8 T cells from SLE patients is diminished.6 An inability to suppress infected cells may lead to ongoing polyclonal B-cell activation and autoantibody production; furthermore, a defect in cell killing may also explain the predisposition of SLE patients towards macrophage activation syndrome. The potential consequences of decreased IL-2 production in SLE are not completely known but are likely to be many, given the many functions of IL-2 in regulating immune responses.