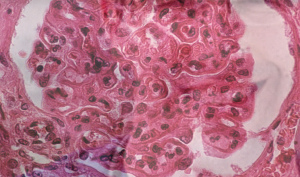
The pattern of lupus glomerular involvement in this patient with lupus nephritis is similar to that in membranous nephropathy, forming wire-loops lesions.
Biophoto Associates/SCIENCESOURCE.COM
The following summary regarding use of tacrolimus (TAC) in lupus nephritis highlights a number of debatable points. Although the role of TAC in lupus nephritis remains unproved for North American populations, it might be an excellent option in some clinical situations. These situations include lupus flare during pregnancy and also for lupus nephritis when the other anchor drugs, such as mycophenolate (MMF) and cyclophosphamide (CYC), have failed. Adding TAC may be especially applicable when rituximab cannot be used as a preferred adjunct therapy.
A summary of data supporting this conclusion follows, in the context of mechanisms of action of TAC in nephritis and risks.
Background: History, Mechanisms & Risks
Calcineurin inhibitors carry historical importance in nephrology. Anti-metabolites, such as the pyrimidine analogue azathioprine, and corticosteroids made renal transplant feasible, but it is the calcineurin inhibitors that advanced the transplant era. Calcineurin inhibitors proved to work well in idiopathic membranous nephritis with nephrotic range proteinuria. Conventionally, it is also thought to be of most value in lupus nephritis when the biopsy reflects a large membranous component.
The ways in which calcineurin inhibitors lead to faster resolution of proteinuria in lupus nephritis include several pleiotropic, non-immunosuppressive, off-target effects. Studies have demonstrated that calcineurin inhibitors cause vasoconstriction of the afferent and efferent glomerular arterioles, that results in decrease in single nephron filtration pressure and, therefore, degree of proteinuria. Additionally, a direct effect upon podocytes by stabilizing podocyte actin cytoskeleton and inhibiting podocyte apoptosis has been postulated.1 Regardless of mechanism, less proteinuria is good—it just may not be equally good for all mechanisms leading to reduced proteinuria.
The most common side effects of calcineurin inhibitors include nephrotoxicity, hypertension, neurotoxicity and metabolic abnormalities. Calcineurin inhibitor nephrotoxicity is manifest either as acute azotemia, which is largely reversible after reducing the dose, or as chronic progressive renal disease, which is usually irreversible. Up to 17% of patients within three years of therapy may have a significant loss of renal function due to drug toxicity, most of which can never be regained.2 TAC can, therefore, be a bridge to somewhere in rheumatology, but not a destination.
Recent Data
New data have reemphasized the importance of proteinuria as a cardinal prognostic feature in lupus nephritis.3 Proteinuria rivals the prognostic value of rising creatinine in conferring risk of eventual end stage of renal disease in lupus nephritis. This emphasis on proteinuria might seem to favor TAC as an adjunct or salvage treatment for lupus nephritis on the grounds that TAC can decrease the degree of proteinuria faster than MMR or CYC. However, most recently—and spectacularly—efforts to cut corticosteroid doses in induction treatment for lupus nephritis using rituximab have had good success.
A British population benefited from a previously unheard of 80% complete response rate.4 Rituximab is, therefore, now on the verge of becoming the best-established alternative or adjunct treatment for lupus nephritis via this steroid-sparing trial of induction. If this observation is confirmed in subsequent trials, it will add much-needed convergent validity.
Previous data supporting rituximab use in lupus nephritis come from French registry data, non-randomized series and meta-analysis.5
All this is despite failure of rituximab to reach its primary endpoint of renal improvement in the only large placebo-controlled trial of rituximab, the LUNAR trial of 2012.6
In 2015, 26 nephrology centers in China reported 368 patients randomized to conventional intravenous CYC vs. MMF plus TAC for induction. At 24 weeks, the MMF plus TAC, called the multitarget regimen, fared better.7 This result implies a substantial benefit conferred by TAC itself, because the general perception is that MMF and CYC are close to equivalent as induction agents in lupus nephritis.
A 2016 report from Hong Kong outlined a five-year, head-to-head comparison showing TAC to be non-inferior to MMF in the role of anchor induction drug for 150 patients with lupus nephritis participating in a balanced randomization.8 In number of flares or relapses over the five years, a non-significant, but sustained, trend favoring MMF emerged.
TAC may be a better choice in pregnant patients than MMF or cyclophosphamide. Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, kidney and nervous system.
Integrating Recent Studies
Any critique of these recent studies featuring TAC and rituximab or an attempt to integrate their data into a proscriptive scheme for prescribing for lupus nephritis must accommodate three caveats. First and most obviously, the transplant rejection context offers more restricted alternatives than the lupus nephritis context.
The second caveat arises out of the recently noted Asian–Hispanic paradox reported in Medicaid data. Gomez-Puerto et al draw attention to the fact that Asian and Hispanic patients with lupus, despite increased incidence, had better prognosis than Caucasians.9 Could Chinese populations or Asian American patients be expected to be better responders to TAC or to other regimens than the African Americans or Caucasians making up most of U.S. populations? Could the MMF-plus-TAC regimen in the Hong Kong report showing MMF and TAC equivalence have been influenced due to the Asian population being at some subtle reduced overall risk from the disease?8
Finally, some TAC response occurs via immediate vasomotor mechanisms occurring before T cell or immunosuppression effects take hold. Would these non-immunologic, non-remission inducing effects of TAC lack durability? One would think so, but the overall benefit of TAC effect does seem durable at two to five years in the Chinese cohort.8,10 This feature of durability, however, may not be generalizable for the same reason that the apparent equivalence of TAC to MMF might not hold in all groups, namely unanticipated differences between populations as implied by the Medicaid survival data showing differences between ethnic groups.9
Proposals for New Studies Comparing TAC with Rituximab
What should the study design be to test the null hypothesis that rituximab and TAC are equivalent in either induction or maintenance? Ideally, half the patients would be African American, for example.
For TAC, the most definitive study would come in the subset for which TAC is most likely to work, that with a strong membranous component on biopsy. This test would also come in the subset with the least chance of suffering TAC nephrotoxicity, that with close-to-normal GFR to start. This means a limited subset of lupus nephritis with class V and histologically mixed classes (IV/V and III/V). It might also mean creatinine less than 1.2 mg/dL for example.
Would a more applicable study of TAC be after failure of MMF alone? But would this study of TAC after failure of MMF need to recruit only the subset of patients with failure of MMF defined as unresponsive or increasing proteinuria without increased creatinine?
Rituximab may need to be included in the control arm because continuing MMF alone would not be ethical if it had already been judged to have failed. Also required would be two types of studies with arms for both drugs: one study to test initial induction and one for salvage after initial failure, MMF plus rituximab vs. MMF plus TAC.
Therefore, we are looking at a narrow window of time for patients with ominous biopsies with membranous component, but good current GFR, in several and different trials to account for both induction and salvage scenarios. These will be hard studies to fill.
Conclusions
Despite newer data suggesting some value in the use of TAC to augment treatment of lupus nephritis, TAC remains salvage therapy for the moment. It remains a particularly good alternative for those concerned about pregnancy and fertility. Despite the well-known negative results of the LUNAR study of 2012, rituximab has gained prominence in competing salvage or steroid-sparing strategy for treatment of lupus nephritis based in part upon recent reports. Given the loss of renal clearance still associated with the calcineurin inhibition and related mechanisms, a hypothetical TAC plus MMF induction strategy for lupus nephritis with membranous component might need to beat or match not only MMF alone, but also the emerging steroid-sparing regimens based on MMF plus rituximab, in order to enter the mainstream.
As further apparent differences between different populations emerge, investigators will have to look to genetics, epigenetic and microbiome differences to explain different response rate for different population groups.11 Clinical readers of studies will have to continue to ask two questions: Do the data support the conclusion (as they do, by and large, for all the studies considered here)? And are these study patients the same as our patients?
Alexey Fomin, MD, is a second-year rheumatology fellow at the University of Louisville in Kentucky.
W. Neal Roberts, MD, is program director and head of the rheumatology division at the University of Louisville.
References
- Liao R, Liu Q, Zheng Z, et al. Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PLoS One. 2015;10(7):e0132724.
- Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003 Sep 4;349(10):931–940.
- Dall’Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: Lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol. 2015 May;67(5):1305–1313.
- Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013 Aug;72(8):1280–1286.
- Weidenbusch M, Römmele C, Schröttle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013 Jan;28(1):106–111.
- Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012 Apr;64(4):1215–1226.
- Liu Z, Zhang H, Liu Z, et al. Multitarget therapy for induction treatment of lupus nephritis: A randomized trial. Ann Intern Med. 2015 Jan 6;162(1):18–26.
- Mok CC, Ying KY, Yim CW, et al. Tacrolimus versus mycophenolate mofetil for induction therapy of lupus nephritis: A randomised controlled trial and long-term follow-up. Ann Rheum Dis. 2016 Jan;75(1):30–36.
- Gómez-Puerta JA, Barbhaiya M, Guan H, et al. Racial/ethnic variation in all-cause mortality among United States Medicaid recipients with systemic lupus erythematosus: A Hispanic and Asian paradox. Arthritis Rheumatol. 2015 Mar;67(3):752–760.
- Yap DY, Ma MK, Mok MM, et al. Long-term data on tacrolimus treatment in lupus nephritis. Rheumatology (Oxford). 2014;53(12):2232–2237.
- Tiniakou E, Costenbader KH, Kriegel MA. Sex-specific environmental influences on the development of autoimmune diseases. Clin Immunol. 2013 Nov;149(2):182–191.
