Why We Need Disease Activity Criteria
1) Disease activity, regardless of the means of its assessment, spans a wide range, which is easier to appreciate if translated accordingly, such as into an activity thermometer;
2) Many agents—particularly the biologics—are indicated for patients with “active” RA, but the term “active” is not well defined (Moderately active? Very active? Very, very active?);
3) Entry criteria for clinical trials require a minimal number of swollen and tender joints (usually at least six to 10), but in clinical practice most patients do not fulfill entry criteria for clinical trials, although these patients suffer from their disease and its progression; and
4) It has been shown in both randomized controlled clinical trials and observational studies that continued active disease—irrespective of the definition of the term “active”—translates into longitudinal progression of joint damage and irreversible functional impairment.2-6
Why Strive for Remission?
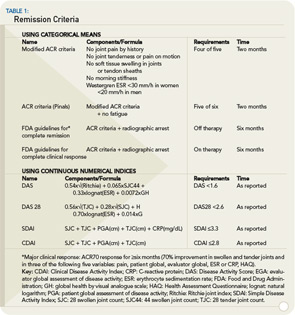
It is well established that disease activity, as measured by acute phase response levels or swollen joint counts over time, correlates with progression of joint destruction.3,4,6,12 We have also learned that individual measures of disease activity do not constitute the most valid and reliable instruments.13,14 As a result, composite indices are preferable because they capture several aspects of disease and translate and combine them into a single score that accounts for the inherent variability of disease between and within the individual patient. Composite disease activity indices, such as the DAS28, the simplified disease activity index (SDAI) and the clinical disease activity index (CDAI) have been increasingly used in clinical trials and practice over the years. (See Table 1) These indices allow for categorization of disease activity as high, moderate, low, and remission by virtue of their scales. While it may seem apparent that a long-term state of high disease activity when compared with a long-term state of remission will lead to different disease outcomes, it is less clear that low disease activity would be associated with a worse outcome than remission, although this is the case.
The cross-sectional data depicted in Figure 1reveal that median Health Assessment Questionnaire (HAQ) scores are quite different when comparing patients at low disease activity with those in remission.15 The long-term analyses shown in Figure 2reveal that low disease activity over time allows for more progression of joint damage than remission over time, and that this is true irrespective of the measure applied.16 If we wish to halt progression of joint damage and achieve maximal reversal of functional impairment, we need to aim for remission. Although there is a possibility that—even in remission—individual patients have some mild progression of joint damage, this is not the case for the vast majority.17 Thus, the lowest degree of disease activity—remission—is the best we can currently aim for to obtain an optimal functional and structural outcome and, therefore, the most desirable state for our patients.