Where We’re Going
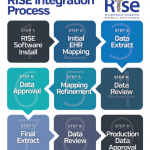
The Summit called together a diverse group of individuals to discuss the rheumatology community’s future needs and how RISE could best meet them. Attendees included clinicians, practice administrators, researchers, rheumatology leaders and more. Based on their input, the ACR mapped the path for the future of RISE, beyond just supporting us in federal reporting and individual quality improvement. Here are the exciting areas into which RISE will now expand.
- Collecting long-term clinical data and patient-reported outcomes data: The need for longitudinal data on our patients is immense, especially with regard to long-term outcomes. These data will serve as one way to validate the importance of access to rheumatologic care to prevent disability and reduce the burden of disease. Likewise, there is a need to meet the growing demand to incorporate patient feedback.
- Characterizing clinical practice patterns, identifying high-quality providers, provider benchmarking: The need for practitioners to learn how they are doing compared with their peers has long been unmet. RISE now has the potential to be used to collect, analyze and report these data, and it is creating a large network of providers who can learn from each other, share valuable experiences and provide mentorship beyond individual practices.
- Quality measure development and testing: RISE can provide the means to validate new quality measures in our specialty. For example, work is already underway to develop risk-adjusted outcome measures rather than just process measures. With RISE, we continue to expand our quality measures portfolio into other disease areas and measurement domains.
- Studying rare diseases and special populations: Through the collection of clinical data on almost 2 million patients to date, the ability to study thousands of patients with a rare disease is now possible. Further, advancements in disease phenotyping using natural language processing to analyze free text would significantly improve our ability to identify patients who are part of special populations or have rare diseases that lack clear diagnosis codes.
- Addressing value-based reimbursement: Based on its success as a tool for federal reporting requirements, we have an opportunity to expand RISE to help rheumatology providers succeed in value-based reimbursement programs from both public and private payers.
Together, the participants in the Summit delved into the ways these areas were important to the rheumatology community and envisioned what expansion into these areas would look like. This invaluable input will lay the groundwork for how RISE will help lead the rheumatology community into the future. RISE, like the call in the courtroom, focuses our efforts to achieve the highest level of evidence-based improvement in the care of our patients.
Participation in the RISE registry is a benefit available to all ACR members without charge. Join now, and see how RISE can help move us all forward in quality improvement and beyond.