 CHICAGO—Temporal artery biopsy (TAB) has long been the gold standard for the diagnosis of giant cell arteritis (GCA). However, with continued advancements in ultrasound resolution and growing provider expertise, temporal artery ultrasound (TAUS) is increasingly being used in practice. This shift is reflected in the differing recommendations of the ACR/VF and EULAR guidelines, with the European guidelines now favoring ultrasound as the initial imaging modality.1,2 In the ACR Convergence 2025 session, Great Debate: Ultrasound vs. Temporal Artery Biopsy in Giant Cell Arteritis, two vasculitis experts went head to head to determine the ideal first test in the diagnosis GCA.
CHICAGO—Temporal artery biopsy (TAB) has long been the gold standard for the diagnosis of giant cell arteritis (GCA). However, with continued advancements in ultrasound resolution and growing provider expertise, temporal artery ultrasound (TAUS) is increasingly being used in practice. This shift is reflected in the differing recommendations of the ACR/VF and EULAR guidelines, with the European guidelines now favoring ultrasound as the initial imaging modality.1,2 In the ACR Convergence 2025 session, Great Debate: Ultrasound vs. Temporal Artery Biopsy in Giant Cell Arteritis, two vasculitis experts went head to head to determine the ideal first test in the diagnosis GCA.
Ultrasound Should Replace Biopsy for GCA Diagnosis
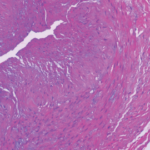
As the first person to describe the ultrasound halo sign in GCA, Wolfgang Schmidt, MD, MACR, Professor of Charité University Medicine at Waldfriede Hospital, Berlin, was well suited to argue his position. He also pioneered the GCA fast-track clinic. Accordingly, he opened his presentation by making it clear he felt strongly about encouraging clinicians to start with ultrasound when diagnosing GCA.
Dr. Schmidt made note of the change in practice patterns over the past two decades, with providers moving from TAB to TAUS. In the updated EULAR guidelines for large-vessel imaging, TAUS is recommended as the first-line modality for GCA diagnosis.2
Using the ACR/EULAR classification criteria for GCA, he demonstrated that ultrasound has greater potential diagnostic yield. Although both positive TAB and TAUS represent five points on the criteria, confirmation of bilateral axillary involvement with ultrasound provides an additional two points toward the diagnosis.3
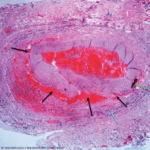
Dr. Schmidt commented on many disadvantages of temporal artery biopsy—the greatest being a delay in diagnosis, resulting in a period of uncertainty, sometimes over five days, and requiring additional patient contact once the results are finalized. Even once the results are in, data suggest that providers do not alter their management. He highlighted a study that demonstrated that even in the setting of a negative biopsy, 87% of patients were continued on prednisone.4 Further, biopsies are invasive procedures involving removal of a portion of the artery, which confers risk of facial palsy and hematoma, although he acknowledged the overall incidence of these events is low.5
Dr. Schmidt contrasted these challenges with the ease of performing TAUS, which only requires about 10 minutes in experienced hands, even when performed bilaterally. With examination of the axillary arteries, ultrasound also has the unique ability to capture the approximately 20% of cases of isolated extra-cranial GCA.6 This begs the question, however: Are we missing other forms of extracranial GCA when only examining the temporal and axillary arteries via ultrasound? The answer appears to be rarely; only two of 72 patients had aortitis or iliac artery vasculitis in a study comparing TAUS with with positron emission tomography/computed tomography (PET-CT.)7