Step 3: Compare Apples to Apples
Whenever we check drug concentrations in a patient, we need to be able to compare this level with some target from the literature to know whether or not it is in a range that is efficacious, toxic or associated with non-adherence. As seen in steps 1 and 2 of this tutorial, it is crucial to compare apples to apples—drug levels at the same dose and taken at the same time, noting whether the sample was drawn at steady state. A few other reasons may explain why drug levels differ across studies, including the assay used to detect the drug, the units of measure (e.g., ng/mL vs. μg/mL) and the matrix/sample the drug is measured in (e.g., whole blood, serum or plasma).
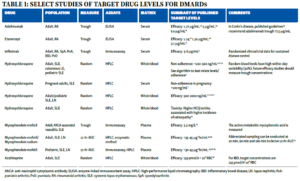
Table 1 summarizes the typical matrix and assay used for various DMARDs. For reference, 1 ng/mL=0.001 mg/L=0.001 μg/mL.
The majority of drug levels are measured in plasma or serum, which generally have similar performance characteristics. However, certain drugs, such as HCQ, partition into red blood cells, meaning whole blood levels are much higher than in plasma or serum and cannot be directly compared. For HCQ, whole blood levels are more precise and are generally preferred for non-pregnant patients.7 Additionally, different assays are used to measure drug levels, and the assays may or may not correlate well with one another. Most small molecule DMARDs can be detected using high-performance liquid chromatography (HPLC). However, assay techniques for biologic drugs are variable and perform differently in the presence of anti-drug antibodies.1
Step 4 (The Last Step): It’s Your Patient
The final step to interpreting a drug level is to individualize the results to the patient in front of you. Individual patients may have drug levels that deviate from expected for a variety of reasons, including medication non-adherence, physiologic alterations in drug pharmacokinetics (e.g., genetics, extremes of body weight, and effects of their disease on organ function), the presence of anti-drug antibodies and drug-drug interactions, among others. Individual interpretation is drug specific and requires a basic understanding of the drug’s pharmacology. For example, HCQ is excreted renally, and blood levels are higher in patients with significant renal disease. As a second example, mycophenolate mofetil drug levels are reduced in the presence of a protonpump inhibitor and if administered with magnesium or aluminum hydroxide antacids.