CHICAGO—In the basic science session, Beyond NETs: Alternative Roles of Neutrophils in Spondyloarthritis, neutrophils are explored as a common pathophysiologic thread among the spondyloarthropathies. In this two-part talk, Dennis McGonagle, PhD, professor of investigative rheumatology at the Leeds Institute of Rheumatic and Musculoskeletal Medicine at St James Hospital, Leeds, England, opens by examining the role…

Does High-Intensity Interval Training Lower Inflammatory Disease Activity?
Experts discuss high-intensity interval training as an underused “disease-modifying drug” for inflammatory diseases. Learn how to safely integrate it into patient treatment plans.

Emerging Treatments in Psoriatic Arthritis
At a scientific session of ACR Convergence 2025, Alexis Ogdie, MD, discussed new and forthcoming treatments for psoriatic arthritis.

What’s New in Psoriatic Arthritis?
Dr. David Pisetsky curated the research presented at ACR Convergence 2025 and answers the question “What new research on psoriatic arthritis has the potential to impact patient care or future research?”

Oral Treatment for PsA Inches Closer to FDA Approval
The FDA will consider a supplemental new drug application for deucravacitinib for the treatment of adults with active psoriatic arthritis based on promising results from clinical trials.

3 AC&R Study Summaries: Avoidable RA Hospitalizations, Psoriasis to Psoriatic Arthritis Transition, & Neighborhood Conditions in Childhood Lupus
New studies reveal predictors for avoidable RA hospitalizations, PsA transition in psoriasis & the impact of neighborhood on childhood lupus.
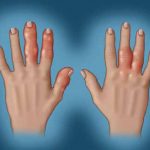
Finding the Panacea: The Management of Psoriatic Arthritis
In a session at EULAR 2025, Dr. Laura Coates discussed the management of PsA, providing insights into the current research and when clinicians may want to consider prescribing specific medications.

FDA Approves Ustekinumab-stba for All Forms & Dosages as Its Reference Product
Ustekinumab-stba (Steqeyma) now has FDA approval for all forms and dosages of its reference product, ustekinumab (Stelara), including a subcutaneous injection to treat pediatric patients with plaque psoriasis or psoriatic arthritis.

In the Details: Diagnosing PsA Requires Drilling Down
Rheumatologists must do some detective work into a patient’s signs and symptoms when considering a psoriatic arthritis diagnosis, according to Philip J. Mease, MD, MACR
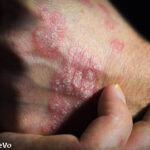
Deucravacitinib Promising for PsA
Results from two studies found that deucravacitinib improved the signs and symptoms of patients with psoriatic arthritis who were biologic disease-modifying anti-rheumatic drug naive and those previously treated with a tumor necrosis factor α inhibitor.
- 1
- 2
- 3
- …
- 17
- Next Page »