(Reuters)—Recro Pharma’s IV meloxicam, a non-opioid injection, did not get approval from the U.S. Food and Drug Administration because the agency said the drug’s pain-relieving effect did not meet its expectations. The company said it plans to meet with the FDA to find solutions. Unlike the drug’s oral version, which has been on the market…
Novartis Receives EU Approval for Infliximab Biosimilar Zessly
ZURICH (Reuters)—Novartis said its Sandoz division received approval from the European Commission for its biosimilar Zessly (infliximab) in gastroenterological, rheumatological and dermatological diseases. Zessly is approved for use in all indications of the reference medicine including rheumatoid arthritis, adult and pediatric Crohn’s disease, adult and pediatric ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis,…
Prenatal TNF Inhibitor Exposure Not Linked to Serious Infections
NEW YORK (Reuters Health)—Children of women with rheumatoid arthritis (RA) who are exposed to tumor necrosis factor-alpha inhibitors (TNFis) in the womb are not at markedly increased risk of serious infections, new findings suggest. “It’s reassuring for mothers who need to take these medications during pregnancy,” Evelyne Vinet, MD, of McGill University Health Center in…
Guselkumab May Best Adalimumab for Psoriasis on the Scalp, Palms & Soles
NEW YORK (Reuters Health)—The interleukin 23 inhibitor guselkumab is associated with more improvement in psoriasis on the scalp, palms and/or soles compared with adalimumab, a new analysis suggests. Andrew Blauvelt, MD, MBA, of Oregon Medical Research Center in Portland conducted a secondary analysis of data from the international VOYAGE 1 and VOYAGE 2 studies, double-blind,…

Alendronate May Provide Cardiovascular Benefits; Plus FDA Approves Subcutaneous Tocilizumab
New research has linked alendronate to reduced cardiovascular death in hip fracture patients…

FDA to Review Abuse-Deterrent Oxycodone Capsule
FDA to Review Abuse-Deterrent Oxycodone Capsule In June, the FDA will discuss the New Drug Application for Remoxy ER at an Advisory Committee meeting. Remoxy ER is a 12-hour, abuse-deterrent, extended-release oxycodone in a capsule formulation.1 The capsule contains a sticky, thick, high-viscosity formulation to deter unapproved drug administration routes, including injection, smoking or snorting….
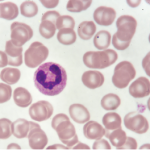
Neutrophil Extracellular Traps & Their Role in Autoimmunity
Certain medications have been associated for decades with the development of drug-induced autoimmunity. New research published in March 2018 in Arthritis & Rheumatology suggests that NETs (neutrophil extracellular traps) are potentially implicated in the mechanisms that lead to drug-induced autoimmunity.1 Peter Grayson, MD, MSc, head of the Vasculitis Translational Research Program at the National Institute…
Study Assesses Immune Checkpoint Inhibitors Safety in Rheumatic Disease
Since they were first introduced in 2011, immune checkpoint inhibitors (ICIs) have become an important treatment for an expanding list of advanced cancers. Some concerns have been raised around the mechanism of action of these immunotherapy agents, making their use in rheumatic diseases (RD) problematical. An article in the March 2018 issue of Arthritis &…
Opioid Refusals: How to Deal with the Angry or Hostile Patient
In July 2017, Todd A. Graham, MD, a practicing orthopedic surgeon in South Bend, Ind., was fatally shot after getting into a heated dispute with a patient and her husband over a requested opioid prescription.1 The murder of Dr. Graham is a tragic example of the potential dangers of physician-patient disagreements. Rheumatology patients often endure…
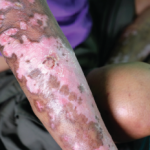
Researchers Test Belimumab in Scleroderma
A yearlong pilot study that evaluated the safety and efficacy of belimumab in a small group of patients with early diffuse systemic sclerosis found no significant difference in the number of adverse events between those treated with the drug and those who received a placebo. Currently, no drugs are approved specifically for the treatment of…
- « Previous Page
- 1
- …
- 52
- 53
- 54
- 55
- 56
- …
- 126
- Next Page »