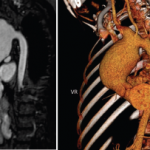
A 13-year-old, adopted girl of unknown ancestry with social anxiety, selective mutism and Takayasu arteritis presented for evaluation of severe, painful, gingival hyperplasia, which limited her oral intake and resulted in weight loss. The young patient was diagnosed with Takayasu arteritis at age 8, when she presented with a persistently elevated erythrocyte sedimentation rate (ESR),…