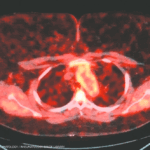
Recent research led to development of a cumulative genetic risk score for Takayasu arteritis, identifying differing susceptibility between groups with different genetic ancestries.

Bryn Nelson, PhD |
Recent research led to development of a cumulative genetic risk score for Takayasu arteritis, identifying differing susceptibility between groups with different genetic ancestries.
Aetna recently notified practices about the launch of its Combined Benefit Management Drug List, which will result in romosozumab-aqqg (Evenity) and infliximab (Remicade) moving to pharmacy-only coverage on July 1. The ACR is working to oppose this change.
This week, the Coalition sent Congressional leadership a letter detailing the results of a recent survey about how underwater biosimilars are impacting physicians’ ability to provide high-quality care. Almost all of the nearly 200 practices queried reported being underwater on several biosimilars, with rituximab and infliximab biosimilars being the most common.

Daniel H. Solomon, MD, MPH, & Andrew Concoff, MD, FACR |
Guest columnists Dr. Daniel Solomon & Dr. Andrew Concoff discuss the potential of technology, such as mobile health apps, to enhance remote care & improve access for underserved patients.

Daniel H. Solomon, MD, MPH, & Andrew Concoff, MD, FACR |
Over our 25 years as rheumatologists, care has advanced greatly. We each completed our rheumatology training in the late 1990s when both infliximab and etanercept first arrived on the U.S. market, ushering in the era of biologics in rheumatology. Since this time, our greater understanding of the immunologic basis of many rheumatic diseases has translated…

Dr. Feely discusses his previous work with the College, his current work as a practicing rheumatologist and how he will lead continued advances on the insurance front.

At a ACR Convergence 2024 session, experts discussed key characteristics, diagnostic challenges & treatment considerations for neurosarcoidosis.

WASHINGTON, D.C.—As of November 2024, there are 16 biologic disease-modifying anti-rheumatic drugs (bDMARDs) that are FDA approved for the treatment of psoriatic arthritis (PsA). Incredible news, right? But as my fellowship program director used to say, “There’s no free lunch.” This buffet of options is excellent for our patients, but poses challenges to the practicing…
The ACR Insurance Subcommittee (ISC) of the Committee on Rheumatologic Care has been hard at work, advocating to payers on behalf of the ACR and its members. Following is an update on some of the work this group has been doing to help address concerns about reimbursement and administrative burden, while ensuring continued access to…

By Matthew A. Sherman, MD, MHSc, & Stacey E. Tarvin, MD, MS |
Why was this study done? Juvenile dermatomyositis (JDM) is the most common type of idiopathic inflammatory myopathy in childhood, and most patients have a chronic disease course requiring prolonged administration of systemic glucocorticoids and immunosuppressive agents. The initial management for patients with moderately severe JDM is relatively standardized, typically including methotrexate and systemic glucocorticoids with…