In a new study, Spiera et al. assessed the safety and efficacy of lenabasum, a synthetic, orally administered agonist of cannabinoid receptor 2 that modulates the endocannabinoid system to activate the resolution phase of innate immune responses, in diffuse cutaneous systemic sclerosis…
Search results for: interstitial lung disease

Rheumatology & Digital Wearables: What’s on the Horizon?
SNOWMASS VILLAGE, COLO.—A major workforce shortage, a population of patients taking immunosuppressants where safety concerns and the patient experience are critical, and an increasing focus on remote patient monitoring and telehealth are driving a discussion regarding the role digital wearables play in rheumatologic care. “We need to be more thoughtful and efficient in taking care…
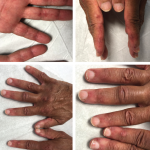
Case Report: A Patient with Clinically Amyotrophic Dermatomyositis & Associated ILD & RA Overlap
Clinically amyotrophic dermatomyositis (CADM), a subset of dermatomyositis (DM), is a rare autoimmune disease characterized by typical DM cutaneous findings (e.g., heliotrope rash, Gottron papules, Gottron sign) without evidence of myositis.1 The incidence of DM and CADM is approximately 9.63 per 1 million people and 2.08 per 1 million people, respectively.2 The association with development…

The Latest Advances in Sjögren’s, Scleroderma, RA, Gout & More
ATLANTA—At the ACR/ARP 2019 Annual Meeting, several widely renowned experts across an array of specialty subjects provided a comprehensive and compelling review of advances in the understanding, diagnosis and treatment of a number of rheumatologic conditions. Sjögren’s Syndrome Frederick Vivino, MD, FACR, chief of rheumatology at Penn Presbyterian Medical Center and professor of clinical medicine…

FDA Update: New Drug Approvals, New & Expanded Indications, & More
ATLANTA—New drug approvals, new and expanded drug indications, and important safety and other updates relevant for rheumatologists were presented by three physicians from the U.S. Food & Drug Administration (FDA) on Nov. 11 at the 2019 ACR/ARP Annual Meeting. New JAK Inhibitor Approved for RA On Aug. 16, 2019, the FDA approved upadacitinib (Rinvoq), an…

Pearls & Myths: Experts Offer Advice & Dispel Myths
GCA, GPA, myositis, new research—rheumatology care keeps clinicians on their toes & requires them to stay up to date…

FDA Rheumatology Update: New Drug Approvals, Plus Expanded Drug Indications & Safety Concerns
Last year, the FDA was busy with new biologic and other drug approvals, new and expanded drug indications, and important safety updates relevant to rheumatology…

Case Report: Obliterative Bronchiolitis in Rheumatoid Arthritis
A 59-year-old woman with rheumatoid arthritis (RA) presented to our pulmonary clinic for progressively worsening dyspnea of five years’ duration. She described progressively worsening dyspnea after a few minutes of walking on level ground. In addition, she noted worsening pain and morning stiffness of the wrists, knees and metacarpophalangeal (MCP) joints, with subcutaneous nodules. She…
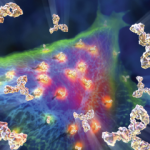
PAD4 Antibodies May Help Predict Treatment Response in Rheumatoid Arthritis
A study published in Arthritis & Rheumatology highlights how the presence of autoantibodies to peptidylarginine deiminase (PAD) may eventually be used to influence treatment decisions in the management of rheumatoid arthritis (RA), sharpening our understanding of disease subtypes.1 Although follow-up prospective studies are needed, these findings underline some intriguing areas for future investigations in immunobiology….

Myositis-Specific Antibodies Identified
The idiopathic inflammatory myopathies (IIM) encompass eight categories: 1) dermatomyositis (DM) in adults, 2) juvenile dermatomyositis, 3) amyopathic DM, 4) cancer-associated DM, 5) polymyositis, 6) immune-mediated necrotizing myopathy, 7) inclusion body myositis, and 8) overlap myositis.1 These categories help classify the myopathies based on clinical and histologic features. The incidence of IIM is estimated at…
- « Previous Page
- 1
- …
- 15
- 16
- 17
- 18
- 19
- …
- 28
- Next Page »