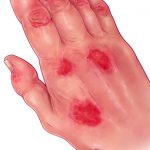
In May, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published its recommendations for the treatment of psoriatic arthritis (PsA).1 The updated recommendations represent advances in drug development and availability since previous recommendations published in 2009, as well changes in treatment paradigms and the importance of associated aspects of the disease.2…