ACR study documents shortage of rheumatologists and predicts greater shortfalls to come
Search results for: psoriatic arthritis

FDA Approves Ustekinumab-stba for All Forms & Dosages as Its Reference Product
Ustekinumab-stba (Steqeyma) now has FDA approval for all forms and dosages of its reference product, ustekinumab (Stelara), including a subcutaneous injection to treat pediatric patients with plaque psoriasis or psoriatic arthritis.

In the Details: Diagnosing PsA Requires Drilling Down
Rheumatologists must do some detective work into a patient’s signs and symptoms when considering a psoriatic arthritis diagnosis, according to Philip J. Mease, MD, MACR
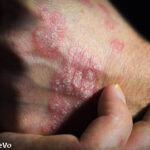
Deucravacitinib Promising for PsA
Results from two studies found that deucravacitinib improved the signs and symptoms of patients with psoriatic arthritis who were biologic disease-modifying anti-rheumatic drug naive and those previously treated with a tumor necrosis factor α inhibitor.

FDA Approval Sought for 2 Pediatric Indications for Guselkumab
The FDA is reviewing supplemental biologics license applications for guselkumab to treat children with juvenile psoriatic arthritis and moderate to severe plaque psoriasis.

FDA Approves Bimekizumab-bkzx (Bimzelx) for 3 New Rheumatic Indications
The FDA has approved bimekizumab-bkzx for the treatment of adults with psoriatic arthritis, non-radiographic axial spondyloarthritis and ankylosing spondylitis.

Are Sacroiliac Joint MRIs Accurate in Routine Clinical Practice?
Hadsbjerg et al. compared assessments of sacroiliac joint MRIs in local MRI reports from routine care in five European countries with re-reads by central experts in patients with a diagnosis of axial spondyloarthritis (axSpA) or psoriatic arthritis (PsA) to estimate the extent of over- or under-reporting of features and misclassification.

Sonelokimab Promising for Patients with PsA
A phase 2 clinical trial has demonstrated the effectiveness for multiple doses of subcutaneous sonelokimab for the treatment of adults with active psoriatic arthritis.

FDA Approves 2 Upadacitinib Formulations for Children with pJIA & PsA
Upadacitinib, as a tablet and oral solution, is now FDA approved to treat children age 2 years and older with active polyarticular juvenile idiopathic arthritis or psoriatic arthritis.

New Indications Possible for Bimekizumab-bkzx
The FDA has accepted applications for three new indications for bimekizumab-bkzx, a humanized interleukin (IL) 17A and IL-17F antagonist: psoriatic arthritis, non-radiographic axial spondyloarthritis and ankylosing spondylitis.
- « Previous Page
- 1
- …
- 14
- 15
- 16
- 17
- 18
- …
- 63
- Next Page »